Department of Endocrinology of Moscow Medical Academy, Moscow, 119992, Russia
The aim of this study was to evaluate parameters of thyroid function in patients with primary hypothyroidism receiving either monotherapy with L-T4 or combination L-T4+L-T3. Fifty-eight women with primary hypothyroidism receiving L-T4 were enrolled in the study. The patients were randomised into two groups: Group 1 (n=42) patients continued monotherapy with L-T4, and Group 2 (n=16) patients were switched to combined therapy with L-T4+L-T3 (25 µg L-T4 was replaced with 12.5 µg L-T3). The final examination was carried out 6 months thereafter. There was also a third group of 20 healthy women (control group). Under monotherapy with L-T4, serum FT4 levels were higher (p <0.05) and FT3 lower (p<0.05) than in the control group, while the monotherapy subgroup of patients with low-normal TSH had serum FT4 levels higher than in the control group (p<0.05). Serum FT4 under combined therapy was significantly lower than in both control and monotherapy groups. FT3 levels did not differ between the two groups of combined and monotherapy subjects; the highest FT3 levels were in the control group. L-T4 replacement therapy is associated with non-physiologically high FT4 and low FT3 levels. Therapy with L-T3 once a day does not simulate the normal production of T3 by the thyroid.
Hypothyroidism, Hormone replacement therapy, Triiodothyronine, Thyrotropin, Thyroxine
INTRODUCTION
Nowadays the main treatment approach for primary hypothyroidism is long-life replacement therapy with L-thyroxine (L-T4). The thyroid produces only a small amount of triiodothyronine (T3), while most of the circulating T3 is produced in peripheral tissues by conversion of thyroxine (T4). Although monotherapy with L-T4 seems to be associated with a quality of life of hypothyroid patients comparable to that enjoyed by healthy people1, combined therapy with both L-T4 and L-T3 has been under investigation over the past few years. Some authors have concluded that the combination of L-T4+L-T3 may provide some advantages over conventional monotherapy with L-T4 in terms of a greater sense of well-being and improved quality of life2,3. The aim of the present study was to evaluate the parameters of thyroid function in patients with primary hypothyroidism receiving either type of replacement therapy.
PATIENTS
Fifty-eight women with primary hypothyroidism following autoimmune thyroiditis (reduced FT4 and increased TSH blood concentrations at the time of initial diagnosis) receiving L-T4 were enrolled in the study. Exclusion criteria were the following: postmenopause, pregnancy, severe concomitant diseases, use of drugs that affect metabolism or bioavailability of thyroid hormones preparations, and history of hyperthyroidism.
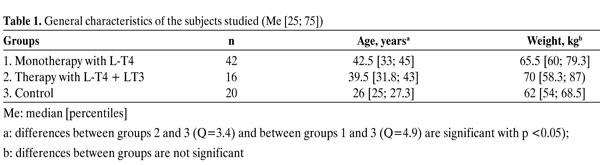
At the beginning of the study, normal TSH levels were maintained in all patients. After randomisation, 42 patients continued monotherapy with L-T4 (Group 1) and 16 started combined therapy with the administration of both L-T4 and L-triiodothyronine (L-T3) (Group 2). During the combined treatment, the dose of L-T4 was reduced by 25-50 µg and replaced with L-T3. The target T4/T3-ratio for the combined replacement therapy of hypothyroidism is not definitively known. With the aim of maintaining normal TSH levels on combined therapy, 25 µg of L-T4 was replaced with 12.5 µg L-T3. Subsequently, in some patients (n= 4) the L-T4 dose was reduced according to TSH level by 25 µg one more time. In most patients additional prescription of 12.5 µg of L-T3 was associated with L-T4 reduction by 25 µg. The final examination in Group 2 was conducted after 6 months of L-T4+L-T3 therapy. The study also included a control group consisting of 20 young healthy women. The patients' characteristics are presented in Table 1
HORMONAL ANALYSIS
Thyroid stimulating hormone (TSH) (normal range 0.4-4.0 mU/l), free thyroxin (FT4) (normal range 11.5-23.2 pmol/l), and free T3 (FT3) (reference range: 3.2-7.2 pmol/l) were measured using chemiluminiscence immunometric assay (IMMULITE, DPC, LA, USA). Blood samples were collected in the morning before administration of thyroid hormones preparations in a fasting state.
STATISTICAL ANALYSIS
"Statistica 6.0" (Stat-Soft, 2001) and "Primer of Biostatistics 4.03" (McGraw Hill, 1998) packages were used for the statistical analysis using the following tests: Mann-Whitney T-test (T) for the comparison of independent samples; Kruskal-Wallis (H) and Dann (Q) tests for the comparison of multiple independent samples; Spearman test (r) for correlation analysis. Data are reported as Me [25; 75] (Me-median; 25 and 75 percentiles). The level of significance was set at 5%.
RESULTS
1. Monotherapy with L-T4
Forty-two patients (Group 1) received standard therapy with L-T4. The compensation of hypothyroidism, indicated by normal TSH level, was achieved by administering 50-125 µg L-T4. The majority of the patients in Group 1 (n= 25) needed 100 µg of L-T4, 7 patients required 125 µg, 9 required 75 µg, and 1 required 50 µg. Although the initial L-T4 dose was calculated on the basis of the patient's weight, the correlation of the final L-T4 dose (which was corrected according to TSH level) with the patient's weight was weak though significant (r= 0.33; p= 0.033).
Three out of the 42 patients (7.1%) of Group 1 had low FT3 concentration (<3.2 pmol/l) despite the fact that all of them had TSH levels lower than 1.0 mU/l. In addition, 5 out of the 42 patients (11.9%) had high serum FT4 level (>23.2 pmol/l) in association with normal TSH and FT3 levels.
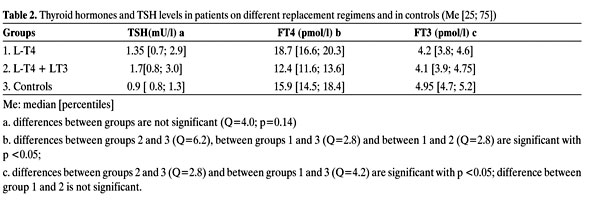
The TSH levels did not differ between the monotherapy and control groups (Table 2, Figure 1). No correlation was found between TSH and FT4 levels in the control group (r= -0.3; p= 0.24), and the correlation between FT4 and FT3 was even weaker (r= 0.23; p= 0.3), which was also true of TSH and FT3 (r= -0.01; p= 0.9). The patients under L-T4 monotherapy had the same correlation results: r= -0.3 (p= 0.07), r= 0.17 (p= 0.3) and r= 0.2 (p= 0.2), respectively. Under monotherapy with L-T4, serum FT4 levels were significantly higher (Q= 2.8; p <0.05) and FT3 levels significantly lower (Q= 4.8; p <0.05) than in the control group (Table 2, Figures 1-3).

Figure 1. TSH levels (mUl) in patients with hypothyroidism on different replacement regimens and in controls (Me [25; 75]).
We analyzed the thyroid function in the subgroups of patients receiving monotherapy with low-normal (<1.5 mU/l) and high-normal (= 1.5 mU/l) TSH levels (Table 3). The cut-off point of 1.5 mU/l was chosen in order to receive comparable sample sizes. In this analysis the following were detected serum FT4 levels in the control group were significantly lower than in the group with low-normal TSH concentration (Q= 3.4; p <0.05), while in the group with high-normal TSH concentration the FT4 levels did not differ significantly from that in the control group (Figures 4 and 5). FT3 levels in the control group were significantly higher than in either subgroup of monotherapy, namely high-normal (Q= 3.0; p <0.05) and low-normal (Q= 4.5; p <0.05) TSH level.
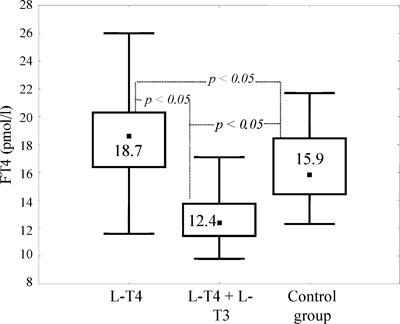
Figure 2. Free T4 levels (pmol/l) in patients with hypothyroidism on different replacement regimens and in controls (Me [25; 75]).
2. Combined therapy with L-T4 and L-T3
Sixteen patients (Group 2) received therapy with combination of L-T4 and L-T3 for 6 months. All of these patients took a constant dose of L-T3 (12.5µg/day) and 50-100 µg/day of L-T4. In this group of patients, the median dose of L-T4 was 75 [75; 81.3] µg/day: 10 patients received 75 µg of L-T4, 4 patients 100 µg and 2 received 50 µg/day. The dose of L-T4 varied in order to maintain normal TSH levels. In most cases 25 µg of L-T4 was substituted by 12.5 µg of L-T3. No one in this group of patients had serum FT3 or FT4 above the normal range. Four patients (25%) had serum FT4 at the lower limit of normal associated with normal FT3 and TSH concentrations.
As shown in Table 2, serum FT4 concentration in patients with L-T4 + L-T3 therapy was significantly lower than in the control group (Q= 6.2; p <0.05) and lower than in the L-T4-monotherapy group (Q= 2.8; p <0.05) (Figure 2). Free T3 levels in the combined therapy group did not differ significantly from those in the monotherapy group. The highest FT3 levels were observed in the control group. (Table 2); it was significantly higher than in the patients undergoing the L-T4 monotherapy (Q= 4.2; p <0.05) and even higher than in patients with combined L-T4 + L-T3 therapy (Q= 2.8; p <0.05).

DISCUSSION
Monotherapy with L-T4
Replacement therapy with L-T4 is a "gold standard" for the treatment of hypothyroidism. In the present study, 42 patients received monotherapy with L-T4. Three of these 42 patients (7.1%) had low FT3 levels despite the fact that all of them had TSH concentrations lower than 1.0 mU/l. Furthermore, 5 of the 42 patients (11.9%) had high FT4 levels associated with normal TSH and FT3 concentrations. In general, serum FT4 levels were significantly higher and FT3 levels were significantly lower under monotherapy with L-T4 than in the control group. Analogous data have also been found by other authors4-7. Thus, according to Igoe et al, normal TSH level was associated with normal FT4 concentration in 70.5% of the patients and normal FT3 level in 88%8. Analogous data were recently published by Mortoglou & Candiloros9. According to Woeber et al, the increased T4:T3 ratio under monotherapy with L-T4 may be caused by the suppression of residual secretion of T3 by the thyroid and decreased T4 conversion7.

Figure 3. Free T3 levels (pmol/l) in patients with hypothyroidism on different replacement regimens and in controls (Me [25; 75]).
It has been suggested that in treating hypothyroidism, the TSH level should be maintained in the low-normal range10. These recommendations are based on the fact that the majority of healthy people have TSH levels between 0.5 and 2.5 mU/l11. We thus evaluated our data on thyroid function in L-T4-monotherapy patients with low-normal (<1.5 mU/l) separately from those with high-normal (³1.5 mU/l) TSH levels (Table 3). The results of this analysis indicated that FT4 levels in the control group were significantly lower than in the patients with the low-normal TSH level, while they did not differ from those in the patients with high-normal TSH (Figure 4). On the other hand, serum FT3 levels in the control group(Figure 5) were significantly higher than in both subgroups of patients on monotherapy with L-T4. It thus seems that an increase of L-T4 dose in order to reach a low-normal TSH level does not generally lead to normalization of FT3 levels in hypothyroid patients. On the other hand, an increase in L-T4 dose may lead to a non-physiological high FT4 in these patients compared to healthy people. Earlier publications have also shown that a L-T4 dose increase leads to further increase of the T4:T3 ratio but not to the normalization of FT3 levels12,13.

Figure 4. Free T4 levels (pmol/l) in patients with hypothyroidism on L-T4 replacement therapy with high-normal and low-normal TSH levels and in controls (Me [25; 75]).
Combined therapy with L-T4 + L-T3
According to our results (Table 2), serum FT4 levels under combined therapy with L-T4 + L-T3 were significantly lower in the hypothyroid group than in the control group and lower than in the monotherapy group (Figures 1-3). Free T3 levels in patients with combined therapy did not differ significantly from those in patients with monotherapy. This can be explained by the fact that in combined therapy a single dose of L-T3 per day every morning is used while blood samples were collected the following morning before taking L-T3, i.e. when FT3 concentration was the lowest because of the short half-life of L-T3. The highest FT3 concentration was seen in the control group (Table 2) in which FT3 was significantly higher than in patients receiving either mono- or combined therapy. Thus, taking L-T3 once a day does not adequately similate the production of this hormone by the thyroid.

Figure 5. Free T3 levels (pmol/l) in patients with hypothyroidism on L-T4 replacement therapy with high-normal and low-normal TSH levels and in controls (Me [25; 75]).
A number of studies have shown that switching of patients with hypothyroidism from L-T4 to the combination of L-T4 + L-T3 results in a reduction of FT4 levels2,14, while FT3 levels increase14,15 or remain unchanged2,16. This is most likely caused by the use of different L-T4 and L-T3 doses since there are no comprehensive recommendations for L-T4 and L-T3 dose calculation for combined therapy. Moreover, it is not clear how the L-T4 dose should be reduced after the addition of L-T3. According to available publications, the additional prescription of 10 µg16 or 12.5 µg2 L-T3 is associated with a reduction of L-T4 by 50 µg. Theoretically, we have to proceed from the understanding that the appropriate ratio of T4 and T3 doses is approximately 5:1 or 4:116. However, in the study of Walsh et al, it was reported that after replacement of 50 µg of L-T4 with 10 µg of L-T3, TSH concentrations increased considerably16. When attempting to use the indicated T4:T3-ratio, we were faced with the same problem. According to Walsh et al16, the increase of TSH level may be caused either by a decrease of T4 level in serum, which is considered to play the main role in the regulation of TSH production, or by the incorrect concept about the required T3:T4-ratio, which is in fact about 4:1 or even 3:1, but not 5:1.
As noted above, one of the main problems with combined replacement therapy is the short half-life of L-T3. This was most likely the reason why FT3 levels in our patients with combined therapy were much lower than in the control group since the blood samples were taken 24 hours post L-T3 administration. In order to avoid such a phenomenon, there have been recommendations14,15 that L-T3 be administered twice a day. However, even if it is appropriate for clinical studies, it would in practice be very inconvenient for the patients and may reduce compliance.
In conclusion, it should be borne in mind that all of the above-mentioned and observed phenomena could be of little clinical significance. This can be judged only by evaluating the actual in vivo effects of different therapeutic approaches, such as life quality or development of side effects related to thyroid hormone insufficiency, or overdosing.
REFERENCES
1. Petersen K, Bengtsson C, Lapidus L, Lindstedt G, Nyström E, 1990 Morbidity, mortality and quality of life for patients treated with levothyroxine. Arch Intern Med 150: 2077-2081.
2. Bunevicius R, Kazanavicius G, Zalinkevicius R, Prange AJ, 1999 Effects of thyroxine as compared with thyroxine plus triiodothyronine in patients with hypothyroidism. New Engl J Med 340: 424-429.
3. Saravanan P, Simmons DJ, Greenwood R, Peters TJ, Dayan CM, 2005 Partial Substitution of Thyroxine (T4) with Tri-Iodothyronine in Patients on T4 Replacement Therapy: Results of a Large Community-Based Randomized Controlled Trial. J Clin Endocrinol Metab 90: 805-812.
4. Salmon D, Rendell M, Williams J, et al, 1982 Chemical hyperthyroidism: serum triiodothyronine levels in clinically euthyroid individuals treated with levothyroxine. Arch Intern Med 142: 571-573.
5. Fish LH, Schwartz HL, Cavanaugh J, Steffes MW, Bantle JP, Oppenheimer JH, 1987 Replacement dose, metabolism, and bioavailability of levothyroxine in the treatment of hypothyroidism. Role of triiodothyronine in pituitary feedback in humans. N Engl J Med 316: 764-770.
6. Ross DS, 2001 Serum thyroid-stimulating hormone measurement for assessment of thyroid function and disease. Endocrinol Metab Clin North Am 30: 245-264.
7. Woeber KA, 2002 Levothyroxine therapy and serum free thyroxine and free triiodothyronine concentrations. J Endocrinol Invest 25: 106-109.
8. Igoe D, Duffy MJ, McKenna TJ, 1992 TSH as an index of L-thyroxine replacement and suppression therapy. Ir J Med Sci 161: 684-686.
9. Mortoglou A, Candiloros H, 2004 The serum triiodothyronine to thyroxine (T3/T4) ratio in various thyroid disorders and after Levothyroxine replacement therapy. Hormones 3: 120-126.
10. Baloch Z, Carayon P, Conte-Devolx B, et al, 2003 Guidelines Committee, National Academy of Clinical Biochemistry. Laboratory medicine practice guidelines. Laboratory support for the diagnosis and monitoring of thyroid disease. Thyroid 13: 3µ126.
11. Hollowell JG, Staehling NW, Flanders WD, et al, 2002 Serum TSH, T(4), and thyroid antibodies in the United States population (1988 t 1994): National Health and Nutrition Examination Survey (NHANES III). J Clin Endocrinol Metab 87: 489-499.
12. Pearce CJ, Himsworth RL, 1984 Total and free thyroid hormone concentrations in patients receiving maintenance replacement treatment with thyroxin. BMJ 288: 693-695.
13. Rendell M, Salmon D, 1985 Chemical hyperthyroidism: the significance of elevated serum thyroxine levels in L-thyroxine treated individuals. Clin Endocrinol 22: 693-700.
14. Clyde PW, Harari AE, Getka EJ, Shakir KM, 2003 Combined levothyroxine plus liothyronine compared with levothyroxine alone in primary hypothyroidism: a randomized controlled trial. JAMA 290: 2952-2958.
15. Levitt A, Silverberg J 2002 T4 plus T3 treatment for hypothyroidism: a double-blind comparison with usual T4. 74th Annual Meeting of the American Thyroid Association (Los Angeles).
16. Walsh JP, Shiels L, Lim EE, et al, 2003 Combined thyroxine/liothyronine treatment does not improve well-being, quality of life, or cognitive function compared to thyroxine alone: a randomized controlled trial in patients with primary hypothyroidism. J Clin Endocrinol Metab 88: 4543-4550.
Address correspondence and requests for reprints to:
Valentin Fadeyev, M.D., Moscow Medical Academy, Department of Endocrinology,
Malaya Trubezkaya 8, Str. 2, Moscow, 119 992, Russia, Tel.: (7-095) 2483833,
Fax: (7-095) 2486477, e-mail: walfad@nccom.ru
Received 28-12-04, Revised 02-02-05, Accepted 01-03-05