Department of Endocrinology, 1George Genimatas General Hospital, Athens, Greece, 2Novartis Hellas, 3St Bartholomew's Hospital, ECIA 7BE, London, UK
Neuroendocrine tumours (NET) have a particular tendency to express functional receptors and/or uptake mechanisms. Radionuclides, such as 111In-pentetreotide, a somatostatin analogue, which bind to somatostatin receptors, present an imaging modality that has been used for both the diagnosis and staging of NET. Scintigraphy with 111In-pentetreotide can identify lesions beyond the diagnostic sensitivity of conventional imaging modalities. In addition, NET that demonstrate positive uptake to a diagnostic 111In-pentetreotide can, in theory, be treated with these radionuclides, thus presenting a novel and evolving therapeutic modality in addition to other traditional therapeutic approaches. Although experience with 90Y-DOTA-D-Phe1-Tyr3-octreotide therapy is still limited, preliminary studies have demonstrated useful activity in a variety of NET with limited side-effects. Large phase II clinical trials using 90Y-DOTA-D-Phe1-Tyr3-octreotide therapy are still on-going and the results are expected to be presented soon. In order to improve the response rates obtained, newer somatostatin analogues are being developed and the combination with other β-emitting particles in addition to 90Y is being considered.
neuroendocrine, tumours, radionuclides
INTRODUCTION
Neuroendocrine tumours (NET) constitute a heterogeneous group of tumours which originate from the diffuse neuroendocrine system (DNES) and are programmed to adopt a specific neuroendocrine phenotype1. The system includes endocrine glands, such as the pituitary, the parathyroids and adrenal glands as well as endocrine islets within glandular tissue (thyroid or pancreatic) and cells disseminated between exocrine cells, such as endocrine cells of the digestive and respiratory tracts2,3. Although NET may vary in clinical presentation, location and specific histology, they share common features which are detected by refined morphological techniques for endocrine granules, histochemical techniques for secretory peptides or amines and electron microscopy3. Some NET may occasionally exert a very aggressive behavior and become highly malignant (poorly-differentiated NET), but the great majority tend to be relatively slow growing (well-differentiated NET) and retain many multipotent differentiation capacities1,3. Such features include the possession of neuroamine uptake mechanisms and/or specific receptors at the cell membrane, such as somatostatin receptors, which can be of great value in identifying and localizing these tumours as well as in their therapy.
111IN-PENTETREOTIDE IN THE DIAGNOSIS OF NEUROENDOCRINE TUMOURS
Radionuclides represent a diagnostic modality where radiolabelled peptide analogues, based on their ability to bind to suitable ligands, have been utilized for the identification and localization of NET4. The use of radionuclides presents an imaging modality based on physiological characteristics (the presence of functioning receptors and uptake mechanisms) rather than on purely anatomical alterations5. This imaging modality has been extensively used for the diagnosis and treatment of NET as the great majority of them express somatostatin receptors and therefore become an ideal target for somatostatin derived radionuclides4,5.
Somatostatin is a 14 aminoacids-peptide hormone that is present in the hypothalamus, the cerebral cortex, the brainstem, the gastrointestinal system and the pancreas, whereas somatostatin receptors have been identified on many cells of neuroendocrine origin6,7. Somatostatin is mainly an inhibitory peptide that has a very short half-life, approximately 2 minutes, which makes it unsuitable for routine clinical use7,8. Introduction of D-amino acids and shortening of the molecule to the bioactive core sequence resulted in the eight amino acids-containing somatostatin analogues1,6,7. Octreotide (Novartis, Basel, Switzerland) is the first somatostatin analogue to be used in clinical practice while considerable experience has also been obtained with lanreotide (Ipsen, Paris, France) and more recently vapreotide (Figure 1)9. These compounds can be conjugated with DOTA (1,4,7,10-tetraazacyclododecane-1,4,7, 10-tetraacetic acid) as a way of coupling somatostatin analogues with various radionuclides5,8. Out of the five subtypes of somatostatin receptors that have been described, somatostatin receptor type 2 is the one most frequently found in NET5,8. Octreotide exerts its highest affinity for somatostatin receptors type 2, 3 and 5 and thus scintigraphy with octreotide reflects the visualization of octreotide-binding somatostatin receptors7,8. In general, "well-differentiated" NET usually express somatostatin receptors which are generally absent from "poorly-differentiated" tumours2,9.
Figure 1. Structure of human somatostatin - 14 and its long acting analogues which are currently used in clinical practice
Covalently linking ethilene-triamine-penta-acetic acid (DTPA) with octreotide results in DTPA-octreotide (pentetreotide); this tracer was initially labelled with 123I, but is more clinically effective with 111In10. Scintigraphy with 111In-pentetreotide has been shown to have a detection rate of 67-91% for all NET but is of low specificity as uptake is also demonstrable in many other tumours, granulomas and autoimmune diseases4,7,11. (Table 1 shows the particular sensitivity of 111In-pentetreotide in the identification and localization of various NET). The fact that abdominal single photon emission computed tomography (SPECT) was not routinely performed in some of the original studies may explain the differences in the detection ability reported between several studies4,8,11,12. Scintigraphy with 111In-pentetreotide is used for the diagnosis and staging of the tumours as well as identifying the patients who may be suitable for treatment with radiolabelled octreotide1,4,7. The detection of an unsuspected lesion in a patient with known metastatic spread usually has little impact on management. In contrast, the detection of such a lesion in patients with a single known lesion or without any known lesion is important in that it may affect the selection of curative surgery, which remains the treatment of choice in patients with NET8. However, there are no clinical or biochemical predictors of a positive scan, and there is no correlation of a positive scan with the anatomical site of the origin of the tumour4,12. In spite of the efforts in developing more specific radioligands, at present 111In-pentetreotide remains the radiopharmaceutical of first choice for the imaging of NET10. The injected activity in adults is 110-220 MBq of 111In-pentetreotide; approximately 80% of i.v. administered radiolabelled 111In-pentetreotide is eliminated via the urinary system10.

BASIC CONCEPTS OF APPLYING RADIOPHARMACEUTICAL THERAPY IN NEUROENDOCRINE TUMOURS
Many tumours are highly radiosensitive, but delivering an adequate dose to the tumour can result in damage to the surrounding healthy tissue. Coupling a radioisotope to a molecule which would specifically bind to tumour cells could, in theory, deliver an effective radiation dose to the tumour without damage to non-tumour tissues, potentially limiting adverse effects8,11,12. The success of the therapeutic strategy depends upon the amount of radioligand which can be concentrated within tumour cells and the rates of internalization, degradation and recycling of both ligand and receptor8,11. Targeted radiotherapy will be effective in tumour treatment if all or nearly all tumour cells have the target antigen, receptor or chemical structure for the radioisotope for the carrier molecule to bind11,13. Tumour heterogeneity will cause incomplete responses at best unless the radiation delivered can kill the nearby tumor cells that are target-negative8,11. Thus, the radioisotope physical properties are important for targeting radiation to the target positive tumour cells but also for the target negative cells as their death will depend on the crossfire from the radioisotope localized on or in the target-positive tumour cells11,13. This non-targeted radiation will also contribute to the radiation dose being absorbed by the surrounding normal cells13. The selection of the best radioisotope to use for target radiotherapy depends on the type of radiation emitted, the emission energies, the distance over which the energy is deposited and the physical half-life of the radioisotope (Table 2)8,11.

Radioiodine for treatment of thyroid disorders has been the best example of a radionuclide targeting agent with a high target-to-nontarget ratio, rapid clearance of the unbound radioisotope and a long residence time in the target11. With the introduction of 131I-labelled metaiodobenzylguanidine (MIBG), the field of radionuclide treatment has been extended to a wide range of NET11,12,14. Metaiodoenzylguanidine is taken up actively by cell membranes and then by neurosecretory granules in the cytoplasm of cells that express amino-uptake mechanisms; more than 90% of pheochromocytomas and paragangliomas, 70% of carcinoids and around 35% of medullary thyroid carcinomas concentrate MIBG2,12,15. Based on a positive scintigraphy with 123I-MIBG, treatment with 131I-MIBG has produced overall objective tumor responses ranging between 7.5-35% in a variety of NET (higher response rates have been obtained in patients with chromaffin cell tumours)2,12,15. As scintigraphy with 111In-pentetreotide is more sensitive than scintigraphy with 123I-MIBG in identifying various types of NET4,12, it is expected that treatment with radiolabelled somatostatin analogues will probably be applicable to a larger population of patients with NET as well as locate the same lesions identified by 131I-MIBG4,12.
RADIOPHARMACEUTICAL THERAPY WITH 111IN OCTREOTIDE
111In emits mainly š-radiation, which passes through tissue relatively easily and can be imaged using a γš-scanner5. In addition, 111In also emits Auger and conversion electrons having particle ranges of 0.02 to 10 µm and 200 to 500 µm respectively and can therefore be used to investigate its antiproliferative effect in malignant tumours8,11. The short ranged Auger electrons are expected to exert an effect on tumour cell proliferation as the radiotoxicity of Auger electrons is very high if their target, the DNA of the cell, is within the particle range8,16. Following initial experiments in rats where a tumouricidal effect of the radiopharmaceutical was established, the treatment was approved for phase I study in humans8,13. Following dosimetry and pharmacokinetic data derived from animal studies, 111In-octreotide was given in humans with at least 2-week intervals between administrations and a total of 8 administrations was aimed at8. Potential hematological, biochemical and endocrine side-effects were examined and patients were scanned 3 and 7 days after each administration of the radiotherapeutical dose. The percentage uptake of the administered dose in total body and in the most prominent tumour was calculated8. With administered doses of 6000 to 7000 MBq, critical organs were shown to be the kidney and the spleen. Thirty end stage patients with NET were treated; twenty-one of these patients received a cumulative dose of 20 GBq [111InDTPA] octreotide8. Seven patients who were treated with a total dose of less than 20GBq had to stop therapy prematurely due to the extension of their disease despite treatment with 111In-DTPA-octreotide8. From the 21 patients who received adequate doses of 111In-DTPA-octreotide (all had progressive disease), 6 showed an antiproliferative effect demonstrated as shrinkage of the tumour. In addition, in another 8 patients treatment with 111In-DTPA-octreotide resulted in stabilization of their disease (Figure 2)8. However, in spite of these moderately encouraging preliminary results, the radionuclides used in receptor scintigraphy, such as 111In, are in general not as suitable for radiotherapy as potent B-emitters, such as yttrium 90 (90Y)8,11-13.
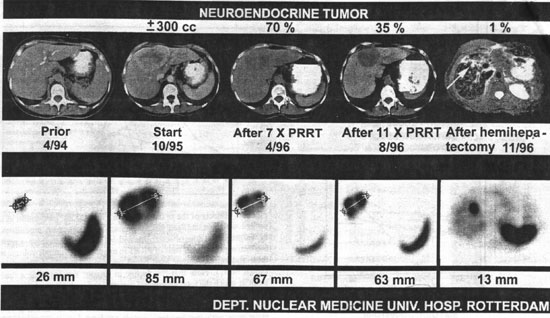
Figure 2. Effects of radiotherapy with [111In-DTPA]octreotide in a patient with a hepatic metastasis of a neuroendocrine tumour treated according to the described protocol. The upper panel shows transversal CT scans, except on 11/96 (MRI), and the lower panel illustrates transversal slices of [111In-DTPA]octreotide SPECT images. The decline in tumour size (300cc) was 30% after seven treatment courses and 65% after 11 courses After the effect of 11 treatment-courses, the option of hemi-hepatectomy became feasible and this procedure was carried out, leaving in situ a lesion of 9mm diameter (MRI) at the cutting-edge (Reproduced with permission from Ann Oncol, 10 (Suppl 2): S23-S29, 1999).
RADIOPHARMACEUTICAL THERAPY WITH 90Y OCTREOTIDE
Radionuclides emitting B-radiation have greater therapeutic potential since the particles they emit have sufficient energy to cause cell damage without penetrating very far into surrounding tissue5,8,11. A high-energy B-emitter attached to a somatostatin receptor analogue could potentially deliver a lethal dose of radiation to a somatostatin receptor-positive tumour with minimal damage to healthy tissue. The same somatostatin analogue (Tyr3-octreotide) used for diagnosis has been coupled to a bifunctional chelating agent (DOTA) for the complexing of 90Y, a pure energetic β-emitter well suited for internal radiotherapy2,8,11-13. In contrast to 90Y-DTPA-octreotide, 90Y-DOTA-D-Phe1-Tyr3-octreotide, (90Y-SMT487, OctreoTherTM), shows no dissociation from the DOTA chelated peptide in serum and can deliver tumouricidal doses of radiation by targeting somatostatin receptor positive tumours who have demonstrated uptake on a diagnostic 111In-pentetreotide scan (Octreoscan)8,13. SMT487 binds with high affinity to somatostatin receptors (subtypes 2 and 3) and retains both its binding properties and physiological function when labelled with 90Y17. OctreoTher induced significant tumour regression in nude mice bearing human pancreatic carcinoma cells and prolonged survival8,17. These good preclinical results led to phase I clinical trials mainly with OctreoTher and various other 90Y-labelled somatostatin analogues17. Phase I clinical trials conducted in patients with NET established that after an intravenous infusion, plasma radioactivity disposition of OctreoTher was multiphasic (terminal t1/2 approximately 8.6h); unbound radioactivity was excreted in urine17. By 24hrs, post-dose plasma radioactivity was <1% of the injected dose while OctreoTher was not significantly taken up by erythrocytes. A correlation between creatinine clearance and total body radioactivity clearance was observed reflecting that the kidney is the principal drug elimination organ. It was therefore suggested that OctreoTher is not recommended in subjects whose creatinine clearance is less than 40ml/min8,17. Radioactivity exposure was mainly, besides the kidneys, to the spleen, urinary bladder wall and tumours. The relative biodistribution and pharmacokinetic profile of OctreoTher was not altered by changes in the absolute peptide dose over the range of 50-500 mcg while concomitant administration of amino acids reduced renal radioactivity uptake without altering tumour uptake in a phase I study17. Cationic amino acids appear to be responsible for the "blocking" of renal tubule uptake of proteins or peptides18. This is because the membranes of renal tubules contain negatively charged sites to which positively charged residues of peptides or proteins are thought to bind8,17,18. An inhibition of this binding site may explain the effects by administering the positively charged amino acid lysine (both D- and L-lysine) on the re-uptake of OctreoTher. A variety of other maneuvers have been attempted, so far without success, in further lowering the renal uptake of OctreoTher8,17.

Figure 3. SPECT image obtained at 24 hours following 90Y-octreotide (OctreoTher) in a patient with a malignant insulinoma demonstrating a liver metastasis.
In another similar preliminary study of 20 patients with a variety of tumours, Paganelli et al. reported results for an escalating dosage using OctreoTher19. Treatment was initiated with 1.1 GBq per cycle (3 cycles per patient), increasing by 0.4 GBq increments. The maximum tolerated dose was not stated but dosimetric estimations showed that the highest doses were delivered to kidneys (3.3 ± 2.2mGy/MBq) and spleen (7.6 ± 6.3 MBq/MBq). Only one patient showed grade II renal toxicity and five had hematological toxicity of the same grade. For higher activities renal or splenic toxicities could be prevented by intravenous administration of D-lysine: the objective response rate (complete + partial responses) was 20%2,19. Phase I clinical trials results support the safety and tolerability of the dose selected for further study. A Novartis-sponsored phase II study involving centers in the USA, Europe and Australia is currently on-going to test the efficacy of OctreoTher in patients with a variety of disseminated NET and the results will soon be published10,17. While in general OctreoTher is a non-šγ emitter, the β-particles can cause localised release of šγ-activity from affected cells ('bremsstrahlung'), and we have found that some imaging of the effectiveness of therapy is possible (Figure 3). A fixed-dose regimen of 120 mCi/cycle x 3 cycles administered with concomitant amino acid infusion has been chosen for the study10,17. Until the results of this study are published, treatment with radiolabelled somatostatin analogues will remain an attractive alternative therapeutic option to current available therapeutic modalities.

Figure 4. Contrast enhanced transaxial computed tomograph images before (top) and after treatment with [90Y-DOTATOC] (bottom) in the second patient. (Reproduced with permission from Lancet, 351:418,1998).
Currently, there is very little experience in using 90Y-DOTATOC, a compound which appears to be identical to OctreoTher, for the treatment of metastatic NET compared to that of 131I-MIBG20,21. 90Y-DOTATOC is a peptide vector DOTA-D-Phe1-Tyr3-Octreotide (DOTATOC; DOTA: 1,4,7,10-tetra-azacyclododecane-N, N',N",N'"-tetraacetic acid), stably labelled with yttrium-9020,21. Out of ten patients with somatostatin receptor-positive metastatic NET treated with 90Y-DOTATOC, the 6 patients who received multiple doses reported improvement in tumour growth, hormonal secretion and symptoms, with minimal side-effects, while the others showed stable disease, suggesting that 90Y-DOTATOC is a potentially valuable therapy for somatostatin receptor-positive tumours (Figure 4)21. In total, 24 patients have been treated with 90Y-DOTATOC and received <7,400 MBq/m2,21. This dose corresponds to 200mCi/m2, and some of the administered cycles were in the presence of concomitant amino acid infusion. Only 4 patients who received doses >360 mCi without concomitant amino acid infusion developed renal toxicity; thus, all doses equal to or below 360 mCi administered with concomitant amino acid infusion appear to have been tolerated safely21.
Studies comparing the detection ability of 123I-MIBG and Octreoscan have consistently shown that Octreoscan is more sensitive than 123I-MIBG and it will also probably locate the same lesions identified by 131I-MIBG4,12. Thus, therapy with either OctreoTher or 90Y-DOTATOC will probably be applicable to a larger population of patients with NET than therapy with 131I-MIBG4,12,19,20. In addition, in occasional patients mainly with hepatic metastases, who show complementary uptake with 123I-MIBG to non-octreotide avid lesions, concomitant use of 131I-MIBG and OctreoTher or 90Y-DOTATOC may be another therapeutic option4,12. As shown from data derived from patients treated with 131I-MIBG, the main predictor of the outcome of therapy with either OctreoTher or 90Y-DOTATOC is principally the extent of the disease and the total tumour load2,15.
In order to overcome the limitations of administering tumouricidal doses of radiotherapy to non-octreotide avid lesions and the lack of uptake to certain areas of the tumours due to tumour heterogeneity several β-emitters, in addition to 90Y, such as 153Sm, 177Lu and 186Re are being considered for therapy when linked to somatostatin analogues (Table 2)11. It has been suggested that for octreotide avid tumours radionuclear therapy with the combination of 177Lu and 90Y will probably be more efficacious than treatment with 90Y alone. In addition, other somatostatin analogues are in development and are expected to show superior imaging and therapeutic properties compared to octreotide.
FUTURE PROSPECTS
It is likely that 'internal radiotherapy' for the treatment of NET will develop considerably within the next few years. Until now, indications with 131I-MIBG have been limited to palliative treatment of malignant chromaffin cell and carcinoid tumours, most often at an advanced disease stage. Therapeutic efficiency has been improved by increasing the injected activity, particularly in patients with limited tumour burden. The recent application of 90Y-labelled somatostatin analogues represents a major advantage as these radionuclides can be taken up by a wider number of NET12. To be fully efficient, these radionuclides should probably be injected into patients with small tumour targets (not more than 1-2cm in diameter). For large macroscopic targets, internal radiotherapy is clearly inefficient because of low and particularly heterogeneous tumour uptake. Although combination therapy with both 131I-MIBG and OctreoTher remains an attractive theoretical option for tumours that demonstrate diagnostic uptake, the only currently established solution is to perform as complete a surgical resection as possible subsequent to injection of diagnostic activity to ensure good uptake of the radiopharmaceutical by tumour targets. If injections are repeated regularly, it is likely that the disease can at least be stabilized for a period of several years if not cured. Finally, it may be hoped that the efficacy of internal radiotherapy will be improved by co-administration of chemotherapeutic drugs whose radiosensitising properties may be synergistic with that of irradiation2.
REFERENCES
1. Arnold R, Frank M, Kadjan U, 1994 Management of gastroenteropancreatic endocrine tumours: the place of somatostatin analogues. Digestion 55: 107-113.
2. Chatal JF, Le Bodic MF, Kraeber-Bodere F, Rousseau C, Resche I, 2000 Nuclear Medicine application for neuroendocrine tumours. World J Surg 24: 1285-1289.
3. Solcia E, Rindi G, Paolotti D, et al, 1999 Clinicopathological profile as a basis for classification of the endocrine tumours of the gastroenteropancreatic tract. Ann Oncol 10: S9-S15.
4. Kaltsas G, Korbonits M, Heintz E, et al, 2001 Comparison of somatostatin analogue and meta-iodobenzylguanidine (MIBG) radionuclides in the diagnosis and localisation of advanced neuroendocrine tumours. J Clin Endocrinol Metab 86:895-902.
5. Lamberts SWJ, Krenning EP, Reubi JC, 1991 The role of somatostatin and its analogues in the diagnosis and treatment of tumours. Endo Rev 12: 561-581.
6. Krenning EP, Kwekkeboom DJ, Pauwels S, Kvols LK, Reubi JC, 1995 Somatostatin receptor scintigraphy. Nucl Med Ann, New York: Raven Press 1-50.
7. Krenning EP, Kwekkeboom DJ, Bakker WH, et al, 1993 Somatostatin receptor scintigraphy with [111InDTPA D Phe1] and [123ityr3] octreotide: the Rotterdam experience with more than 1000 patients. Eur J Nucl Med 20: 716-731.
8. Krenning EP, De Jong M, Kooij PPM, et al, 1999 Radiolabelled somatostatin analogue(s) for peptide receptor scintigraphy and radionuclide therapy. Ann Oncol 10: S23-S29.
9. Kaltsas GA, Mukherjee JJ, Plowman PN, Grossman AB, 2001 The role of chemotherapy in the non-surgical management of malignant neuroendocrine tumours. Clin Endocrinol 55: 575-587.
10. Borbardieri E, Maccauro M, de Deckere E, Savelli G, Chiti A, 2001 Nuclear medicine imaging of neuroendocrine tumours. Ann Oncol 12: S51-S61.
11. Wiseman GA, Kvols LK, 1995 Therapy of neuroendocrine tumours with radiolabelled MIBG and somatostatin analogues. Semin Nucl Medicine Vol XXV 3): 272-278.
12. Kaltsas GA, Mukherjee JJ, Grossman AB, 2001 The value of radiolabelled MIBG and octreotide in the diagnosis and management of neuroendocrine tumours. Ann Oncol 12: S47-S50.
13. De Jonk M, Bakker WH, Krenning EP, et al, 1997 Yttrium-90 and Indium-111 labelling, receptor binding and biodistribution of [DOTA,D-Phe1,Tyr3] octreotide, a promising somatostatin analogue for radionuclide therapy. Eur J Nuc Med 24: 368-371.
14. Taal BG, Hoefnagel CA, Valdes Olmos RA, Boot H, 1996 Combined diagnostic imaging with 131I-MIBG and 111In-pentetreotide in carcinoid tumours. Eur J Cancer 32:1924-1932.
15. Mukherjee JJ, Kaltsas GA, Islam N, Plowman PN, Hikmat J, Btitton KE, Reznek RH, Jenkins PJ, Chew SL, Monson JP, Besser GM, Grossman AB, 2001 Treatment of metastatic carcinoids, phaeochromocytomas, paragangliomas and medullary thyroid carcinoma of the thyroid with 131I-metaiodobenzylguanidine. Clin Endocrinol 55:47-60.
16. Adelstein SJ, 1993 The Auger process: A therapeutic promise? AJR 160: 707-713.
17. Smith CM, Liu J, Chen T, et al, 2000 OctreoTherTM: ongoing early clinical development of a somatostatin-receptor-targeted radionuclide antineoplastic therapy. Digestion 62:69-72.
18. Hammond PJ, Wade AF, Gwilliam ME, et al, 1993 Amino acid infusion blocks renal tubular uptake of an indium-labelled somatostatin analogue. Br J Cancer 67:1437-1439.
19. Paganelli P, Zoboli S, Cremonesi HR, Chinol M, 1999 Receptor mediated radionuclide therapy with 90Y-DOTA-D-Phe1-Tyr3-octreotide: preliminary report in cancer patients. Cancer Biother. Pharmacol. 14: 477
20. Macke HR, Behe M, Froidevaux S, et al, 1997 DOTA-D-Phe 1)-Tyr 3)-octreotide (DOTATOC): a unique somatostatin receptor ligand for labelling with a variety of metalic radionuclides. J Nuc Med 38 (Suppl): 18P (Abs. 57).
21. Otte A, Mueller-Brand J, Dellas S, et al, 1998 Ytrium-90-DOTA-octreotide treatment of somatostatin receptor positive tumours. Lancet 351: 417-418.
Address correspondence and requests for reprints to:
Dr. Gregory Kaltsas, George Genimatas General Hospital,
Athens, Greece, Tel: 003 010 7796043, Fax: 003 010 7779146,
E-mail: gakaltsas@mds.qmw.ac.uk
Received 14-03-02, Revised 29-04-02, Accepted 30-05-02