1Endocrine and Metabolic Bone Disorders Unit, 2nd Department of Internal Medicine and Research Institute and Diabetes Center, Attikon University Hospital, Athens, Greece; 2First Department of Pediatrics and Choremeion Research Laboratory, Athens, Greece; 3Unit of Translational and Clinical Research in Endocrinology, Medical School, National and Kapodistrian University of Athens, Athens, Greece; 4BIOTEKNA Biomedical Technologies, Venice, Italy
BACKGROUND-OBJECTIVES:
We aimed at evaluating the efficiency of a newly developed, advanced
Bioimpedance Analysis (BIA-ACC®) device as a screening tool for
determining the degree of obesity and osteosarcopenia in postmenopausal women
with normal or decreased bone density determined by Dual-Energy X-Ray absorptiometry
(DEXA) in a representative sample of Greek postmenopausal women.
METHODS: This
is a single-gate cross-sectional study of body composition measured by BIA-ACC®
and DEXA. Postmenopausal females with BMI ranging from 18.5 to 40 kg/m2 were
subjected to two consecutive measurements of DEXA and BIA-ACC®
within 5-10 minutes of each other. We used Pearson’s co-efficient to examine
linear correlations, the intraclass correlation co-efficient (ICC) to test
reliability, Bland-Atman plots to assess bias and Deming regressions to
establish the agreement in parameters measured by BIA-ACC® and DEXA.
Last, we used ANOVA, with Bonferroni correction and Dunnett T3 post hoc
tests, for assessing the differences between quantitative and Pearson’s x2
between qualitative variables.
SAMPLE AND RESULTS: Our sample consisted of 84
overweight/obese postmenopausal women, aged 39-83 years, of whom 22 had normal
bone density, 38 had osteopenia and 24 had osteoporosis based on DEXA
measurements, using quota sampling. ICCs and Deming regressions showed strong
agreement between BIA-ACC® and DEXA and demonstrated minimal
proportional differences of no apparent clinical significance. Bland-Altman
plots indicated minimal biases. Fat, skeletal and bone mass measured by BIA-ACC®
and DEXA were increased in the non-osteopenic/non-osteoporotic women compared
with those of the osteopenic and osteoporotic groups.
CONCLUSIONS: BIA-ACC®
is a rapid, bloodless and useful screening tool for determining body
composition adiposity
and presence of osteo-sarcopenic features in
postmenopausal women. Women with osteopenia and osteoporosis evaluated by DEXA
had decreased fat, skeletal and bone mass compared with normal bone density
women, suggesting concordance in the change of these three organ masses in
postmenopausal women.
BIA-ACC®, Bio-impedance, DEXA, Body composition, Fat mass, Menopause, Reproducibility
INTRODUCTION
Menopause constitutes a critical time in a woman’s life, defining the end of her reproductive years and the onset of various metabolic changes, due mainly to the loss of estrogens.1,2 In postmenopausal women, remodelling of body composition take place, i.e. qualitative and quantitative changes in fat, muscle and bone mass, accompanied by emotional and psychological problems;3,4 these changes appear to mediate menopause-related metabolic and cardiovascular morbidity and mortality.1,2,5 Increased total fat mass, trunk fat accumulation and decreased peripheral fat deposition as well as ectopic fat storage are observed and are often associated with adverse health consequences.5-7 In addition, the loss of muscle mass—defined as sarcopenia—in the postmenopausal period and in later life is associated with metabolic dysregulation.6 Hence, the detailed evaluation of body composition, especially fat, muscle and bone mass, and/or their regional distribution, may constitute an important screening test helping to differentiate between high- and low-risk endophenotypes of obesity, sarcopenia and osteopenia/osteoporosis.
Several methods have been employed for assessing total and regional fat mass in postmenopausal women.8 Anthropometric variables, such as BMI, waist circumference, waist-to-hip ratio, waist-to-thigh ratio or skinfold thickness, have been widely used in various research or clinical settings; generally, these methods are characterized by limited accuracy and/or are subject to large intra- and inter-observer variability.8,9 Various methods, such as underwater weighing and deuterium oxide (D2O) dilution, have been proposed for body composition analysis, but are expensive or else time-consuming, are not patient-friendly and are restricted to research settings. Computed tomography10 and magnetic resonance imaging (MRI) are currently the “gold standard” methods for the direct measurements of total and regional fat mass; however, their routine use is limited because of inaccessibility to equipment, relatively high cost and, in the case of computed tomography (CT), exposure to ionizing radiation. Dual energy X-ray absorptiometry (DEXA) represents a reliable alternative method of total and regional fat mass estimation because its use offers significant advantages over both CT and MRI, including improved feasibility, lower cost, minimal radiation exposure, high accuracy and reproducibility.8,11
Bio-impedance (BIA) devices represent an effective alternative solution to inaccessibility and are likely to prove efficient for fat mass assessment in the clinical setting. BIA-ACC® is an advanced BIA device that provides quantitative and qualitative information about body composition, including analyses at the molecular and cellular level;12 the same method reveals associations of body composition parameters via medically unexplained symptoms (MUS) and a Physical Activity Rating (PA-R) questionnaire.13 This device uses proprietary algorithms for reporting risk factor estimations, such as fat, skeletal muscle and bone mass, intramuscular fat mass, as well as presence of systemic inflammation.13-15 BIA-ACC® is a bloodless, simple, inexpensive and rapid method that does not require skilled staff and, most importantly, does not expose individuals to any radiation.
In this single-gate reproducibility study, we aimed to assess the reliability and agreement between measurements of BIA-ACC® and DXA; we assessed the BIA-ACC® device’s diagnostic abilities in relation to fat, skeletal muscle and bone mass; finally, we evaluated the clinical significance of MUS and PA-R, as opposed to body composition, in a representative sample of Greek postmenopausal women with or without bone disorders.
METHODS
Procedure
The protocol of this study16 conformed to the Declaration of Helsinki and was approved by the Institutional Biomedical Research Ethics Committee of the Medical School of the University of Athens. Informed consent was granted by all participants before enrolment in the study.
This study was conducted from October to December 2015. We evaluated participants who presented for DXA bone density assessment at the Endocrine and Bone Metabolic Disorders Unit of the 2nd Department of Internal Medicine of the University of Athens, at the Attikon University Hospital, Athens, Greece. We performed two measurements. First the participants were evaluated by dual energy X-ray absorptiometry (Hologic QDR series Discovery W densitometer of fan beam technology, Hologic, Bedford, MA, USA, provided with software APEX, version 3.2) for bone density assessment (spine, left hip) and whole-body composition scans (whole body DXA scans). Within 5-10 minutes, body composition was also evaluated by using a bio-impedance portable device (BIA-ACC©, BIOTEKNA, Inc., Venice, Italy). The BIA-ACC® device provides information on total body water, extracellular, intracellular water, fat mass (FM) and fat free mass (FFM) measured both in kilograms (kgs) and as body weight percentage (BW %), bone mass (BM) in kg, skeletal muscle mass (SM) in kgs and as fat free mass percentage (FFM %), etc. (Supplemental Material). Reporting of the study conforms to the GRAAS Statement and the broader EQUATOR guidelines.17
Definitions-Participants-Sampling
Menopause was defined as 12 months of amenorrhea and follicle stimulating hormone serum concentrations >30 U/l. Premature menopause was defined as 12 months of amenorrhea before the age of 40. The T-scores of the bone density measurements, which compare the condition of the individual’s bones with those of an average young person with healthy bones, were taken into consideration, as the subject group comprised postmenopausal women. T-scores >-1 were considered normal, -1 to -2.5 defined osteopenia and ≤-2.5 defined osteoporosis.18 Inclusion criteria included absence of any life-threatening disease (e.g., HIV infection, cancer); presence of any disease that could affect body fat mass; epilepsy; other neurological or severe mental disorders; endocrine or genetic disorders; morbid obesity; or lipodystrophy. Exclusion criteria included presence of metal devices, such as orthopedic prostheses or heart pacemakers, and atopic dermatitis that could cause irritation from skin patches. All women reported a relatively stable body weight (±5%) within the previous 6 months. We used quota sampling to provide a good approximation of the population and to offer better assurance against sampling bias. Moreover, since the parameters of validation in reproducibility studies are influenced by the large (>100) number of participants, we chose to include an adequate number of participants, but not exceedingly large, to scrutinize our results.19,20
Anthropometry
All anthropometric variables were measured by the same trained physician and operator. Body weight and standing height were measured in light clothing and no shoes using a digital scale with an accuracy of 0.1 kg, and an attached stadiometer (Seca 769; Seca Ltd., Vogel & Halke, Hamburg, Germany) to the nearest 0.1 cm, respectively. BMI was calculated as body weight in kg divided by height in meters squared (Kg/m2), and cut-off points were as set by the World Health Organization (WHO). Women with BMI ≥30 were defined as obese and those with BMI <30 were defined as non-obese.7,21
DEXA-derived indices
As the reference method, DEXA scan (Hologic QDR series Discovery W densitometer of fan beam technology, Hologic, Bedford, MA, USA, provided with software APEX, version 3.2) was used for the measurement of bone density and of whole and regional body composition estimates, including fat and fat free mass. DEXA provides estimates by measuring the body’s absorbance of X-rays at two different energies, given that fat, bone mineral and fat-free soft tissue have different absorption properties. The subjects were positioned for regional and whole-body scans, according to the manufacturer’s protocol. Thus, they lay in a supine position on the scanner table with straightened legs and with their arms close to the body. They were instructed to remain as still as possible during the scan. In parallel with bone density, whole-body composition analysis provided data on total FM and its regional (trunk, arms, legs, head) distribution, with an average time of measurement of approximately 7 minutes (QDR bone densitometer with fan-beam technology, software version for Windows XP 12.3, Hologic Discovery-W, Bedford, MA, USA). To ensure proper function of the QDR system daily quality control procedures were performed. We obtained in vitro precision using an appropriate spine phantom of the manufacturer daily and a step phantom on a weekly basis as well. In vivo precision was achieved using repeated measurements after repositioning of the same patient: this was 0.9% for the lumbar spine, 1.1% for total hip, 1.5% for fat %, 1.9% for fat mass and 0.9% for free fat mass.
BIA-derived indices
Bio-impedance body composition analysis was performed using a portable BIA device (BIA-ACC®, BIOTEKNA, Inc., Venice, Italy) within 5-10 minutes after the DEXA measurement. This device applies alternating currents using two different frequencies, 50 and 1.5 kHz (bi-frequency measurement method), to measure body composition, based on a multi-compartment model (2C, 3C, 4C, 5C).12 The subjects lay supine on an examination bed, while there was no skin contact with metallic elements. Two skin patches—with a horizontal distance of 10 cm between them—were applied on the dorsal surface of the right hand and an additional pair of skin patches on the dorsal surface of the right foot (Hand-to-Foot). The skin patches were connected to the electrodes of the BIA-ACC® device. The formulas used for computations have been described in detail elsewhere.13
BIA-ACC® measures additional indices
Immediately before the BIA measurement, the participants were asked to fill in two questionnaires: a MUS close-ended questionnaire inherent in the BIOTEKNA platform program of a personal computer—responding ‘yes’ or ‘no’—and a Physical Activity Rating questionnaire (Supplemental Material).22 Information about body composition was provided at the quantitative and qualitative molecular, cellular and functional levels. The MUS analyses were presented in a 3-page PDF file.12 The device uses proprietary algorithms for reporting various body composition risk factors for health, such as BM, FM, intramuscular FM (IMAT) and systemic inflammation (Supplemental Material).13
Lastly, the BIA-ACC® device generates reports of a SM index. Sarcopenia was defined by SM index calculations obtained by dividing appendicular SM evaluated by DEXA divided by body height squared (ASM/Ht2), and the percentage of SM index (SMI%=total SM/body mass × 100) defining Class I sarcopenia in females as 19-24% and Class II sarcopenia as below 19% of total body weight.14,23,24 The DEXA and BIA-ACC® measurements were performed by the same two operators, respectively.
Statistical analyses
For all analyses, p <0.05 was considered statistically significant. The normality of data was assessed with the Shapiro-Wilk test and normal probability plots (Q-Q plots, P-P plots). BIA FM in kgs and as BW % were classified as index tests. We used intraclass correlation co-efficient (ICC)25 to assess the within-subjects variance of the FM, either in kgs or in body weight percentage (BW%), Deming regressions26 to assess the agreement between the measurements of the two methods and Bland-Altman plots to quantify the bias and the range of agreement within which 95% of the differences between the two types of measurement were included.27 We performed calculations in order to evaluate BIA-ACC®-determined bone mass as a screening test for abnormal T-score.28 Pearson’s chi squared tests (x2) were used for the evaluation of association between qualitative variables, Pearson’s correlation co-efficient to assess the correlations between the quantitative variables and one-way variable analyses (ANOVAs), using Bonferroni correction, and Dunnett’s T3 post hoc tests to assess the differences between the quantitative variables. We performed all statistical analyses with SPSS v.21 and MedCalc v.14.
RESULTS
Participants
The selection process is described in Figure 1 and the characteristics of subjects are presented in Table 1. The study group consisted of 84 postmenopausal women aged 39-83 years, with BMI ranging from 20.8 to 38. Among them, 22 females had normal bone density, 38 were diagnosed with osteopenia and 24 with osteoporosis, based on the T-scores of the DEXA measurements.
Figure 1. Flowchart/selection process of the participants of the study.
The mean values of the estimates by BIA-ACC® and DEXA devices are presented in Table 2. Statistically significant differences were found in weight, BMI, DEXA FM in kgs, DEXA FFM in kgs, BIA FM in kgs, BIA IMAT in kgs, BIA SM in kgs, BIA SM as FFM %, BIA-ACC® BM in kgs and mineral mass of bones as kgs (Table 2). Post hoc comparisons revealed that the normal bone density group exhibited increased BIA SM in kgs when compared with the osteopenia (p=0.012) and osteoporosis groups (p=0.003), increased BIA SM as FFM % when compared with the other two groups (p=0.019; p=0.004, respectively), increased BIA BM in kgs (p=0.046; p=0.012, respectively) and increased BIA mineral mass of bones (p=0.046; p=0.012, respectively). Post hoc comparisons of DEXA FFM and FM in kgs revealed increased FM in the normal density group when compared with the osteoporosis group and increased FFM when compared with the other two groups. Lastly, the normal bone density group exhibited increased IMAT when compared to the osteoporosis group.
Strong associations were demonstrated between the three groups and the presence of sarcopenia as estimated by BIA-ACC® and the bone mass as risk factor as estimated by BIA-ACC®. When the three-dimensional group variable (normal bone density group, osteopenia, osteoporosis groups, estimated by DEXA) was transformed into a two-dimensional group variable (Normal T-scores; Abnormal T-scores estimated by DEXA), the strong relation between the bone, as risk factor, and the two-dimensional group variable was verified (x2=10.219; df=1; p <0.001; Pearson’s contingency co-efficient=0.329; p <0.001). We also found strong relations between the 2-variable groups (Normal T-scores/Abnormal T-scores) and the presence of sarcopenia (Yes/No) (x2=5.476; df=1; p=0.019), the presence of mood disorders (x2=5.671; df=1; p=0.017) and feelings of guilt (x2=5.255; df=1; p=0.022).
Height of the study participants was negatively correlated with age (r=-0.469; n=84; p <0.001), not only in the total sample but also in the normal bone density group (r=-0.432; n=22; p=0.045), the osteopenia group (r=-0.455; n=38; p=0.004) and the osteoporosis group (r =-0.518; n=24; p=0.01).
Statistically significant correlations were observed between fat mass, muscle mass and bone mass in kgs. In the total sample, bone vs. skeletal muscle mass in kgs (r=0.99; p <0.001) and bone vs. fat mass in kgs (r=0.634; p <0.001) were significantly and strongly correlated. The same trend of strong, statistically significant linear correlation was observed between muscle mass and fat mass in kgs (r=0.652; p <0.001). The corresponding correlations between bone and skeletal muscle mass in kgs in the three groups were strong and statistically significant (Normal bone density group: r=0.991; p <0.001; Osteopenia: r=0.99; p <0.001; Osteoporosis: r=0.983; p <0.001). The correlations between bone and fat mass in kgs were statistically significant in the normal bone density group (r=0.56; p=0.007) and in the osteopenia group (r=0.733; p <0.001) but not in the osteoporosis group. The same finding was observed in the correlations between fat and skeletal muscle mass (Normal bone density: r=0.549; p=0.008; Osteopenia: r=0.785; p <0.001) (Figure 2).
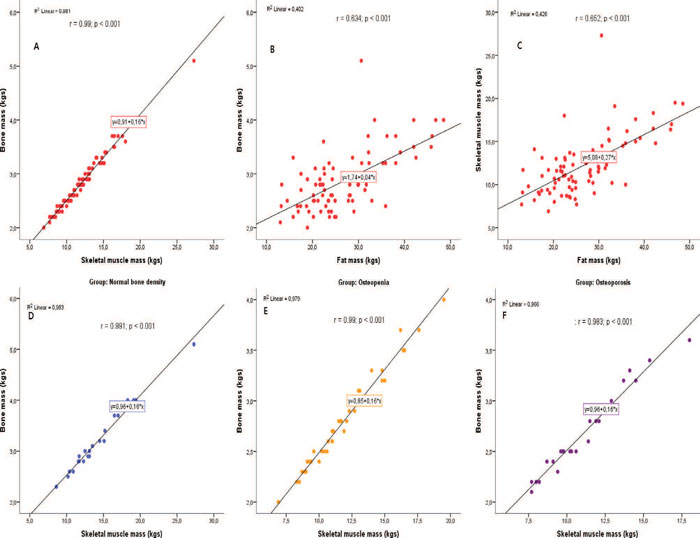
Figure 2. Important Pearson’s correlations observed in the study.
Index Test results
In this study, BIA-ACC® fat and fat free mass in kgs and as BW% were the index tests. For the total sample, BIA-ACC® fat mass exhibited strong positive linear correlations with the DEXA FMs, either in kg or as BW % (Supplemental Material).
The reliability of BIA-ACC® fat mass results proved to be almost perfect for DEXA fat mass either in kgs [F(83)=38.402; ICC=0.949; 95% C.I.: 0.923 – 0.967] or BW % [F(83)=7.425; ICC=0.763; 95% C.I.: 0.656 – 0.839].
We performed Deming regression plots of BIA-ACC®/DEXA fat mass in kgs (Intercept: -4.7312; S.E.: 1.3274; 95% CI: -7.3715 to -2.0910/Slope: 1.0393; S.E.: 0.04574; 95% CI: 0.9484 to 1.1303) and as BW % (Intercept: -14.0770; S.E.: 5.6619; 95% CI: -25.3383 to -2.8157/Slope: 1.2025; S.E.: 0.1273; 95% CI: 0.9492 to 1.4558) (Figure 3) and of BIA-ACC®/DEXA Fat Free Mass in kgs (Intercept: 0.4425; S.E.: 6.7195; 95% CI: -12.9222 to 13.8073/ Slope: 1.0630; S.E.: 0.1684; 95% CI: 0.7282 to 1.3979) and as BW% (Intercept: -4.4528; S.E.: 6.4840; 95% CI: -17.3492 to 8.4437/Slope: 1.1707; S.E.: 0.1158; 95% CI: 0.9403 to 1.4010) (Figure 4). The Bland-Altman plots for BIA-ACC® fat mass and DEXA fat mass in kgs (mean fat mass in kgs difference=3.5; SD=2.59; 95% CI=2.9 to 4.09; p <0.001; Lower limit=-1.54; 95% CI=-2.5 to -0.57; Upper limit=8.61; 95% CI=7.65 to 9.58) and as BW % (mean fat mass as BW% difference=5.27; SD=3.86; 95% CI=4.43 to 6.11; p <0.001; Lower limit=-2.29; 95% CI=-3.73 to -0.85; Upper limit=12.84; 95% CI=11.40 to 14.27) were acceptable (Figure 5). Bland-Altman plots for FFM in kgs (mean fat free mass in kgs difference=-2.86; SD=4.21; 95% CI=-3.78 to -1.95; p <0.001; Lower limit=-11.13; 95% CI=-12.70 to -9.56; Upper limit=5.39; 95% CI=3.82 to 6.96) and FFM as BW% (mean fat free mass as BW% difference=-5.19; SD=3.72; 95% CI=4.43 to 6.11; p <0.001; Lower limit=-12.49; 95% CI=-13.88 to -11.11; Upper limit=2.11; 95% CI=0.72 to 3.49) were also acceptable (Figure 6). Bone mass as a risk factor for osteopenia/osteoporosis, determined by the BIA-ACC® device, proved promising in predicting abnormal T-score presence by DEXA (Table 3). The ratio of fat mass over skeletal muscle mass was stable in the non-osteoporotic, osteopenic and osteoporotic subjects (Figure 7). However, there was a positive correlation of BMI or fat mass with IMAT in all three groups.

Figure 3. Deming regression of BIA-ACC® vs. hologic fat mass in kgs measurements; Deming regression of BIA-ACC® vs. hologic fat mass as body weight percentage measurements.
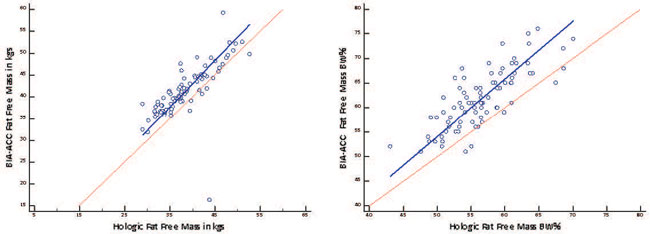
Figure 4. Deming regression of BIA-ACC® vs. hologic fat free mass in kgs measurements; Deming regression of BIA-ACC® vs. hologic fat free mass as body weight percentage measurements.

Figure 5. Bland-Altman plot for the difference between hologic fat mass in kgs and BIA-ACC fat; Bland-Altman plot for the difference between hologic fat mass in kgs and BIA-ACC Fat.

Figure 6. Bland-Altman plot for the difference between hologic fat free mass in kgs and BIA-ACC fat; Bland-Altman plot for the difference between hologic fat free mass in kgs and BIA-ACC fat.
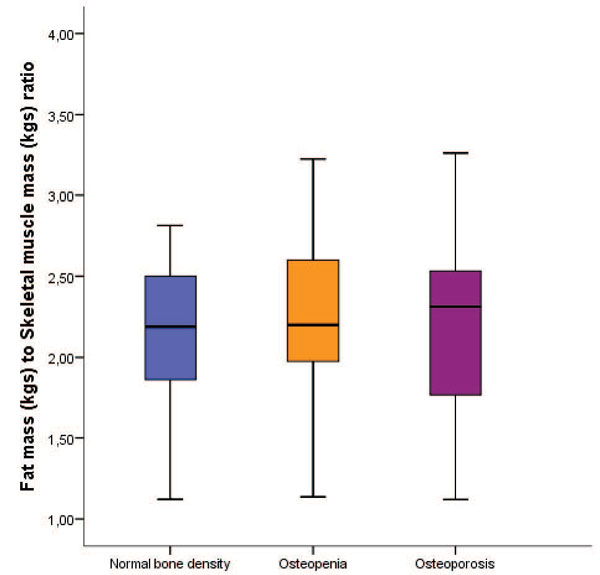
Figure 7. Fat mass in kgs to skeletal muscle mass in kgs ratio shown as a marker of concordance between fat and muscle mass and absence of frailty in the three groups. Of note, intramuscular adipose tissue (IMAT) was directly proportional to BMI and fat mass, suggesting poor muscle functioning in the more obese subjects of all three groups.
DISCUSSION
We found that BIA-ACC® measurements had high reliability and a relatively strong agreement with DEXA measurements in evaluating body composition variables in a representative sample of postmenopausal women. Our subjects were lean, overweight or obese. Of note, subjects with normal bone density demonstrated increased BMI, FM in kgs and as BW %, by both BIA-ACC® and DEXA, when compared with the other two groups, indicating a possibly protective role of obesity and increased body weight in osteoporosis development, in accordance with other studies.29,30 Increased weight-related changes in body composition, metabolic and hormonal factors or the observed decline in physical activity might provide pathophysiologic mechanisms for populations prone to gain fat mass and lose muscle and bone mass.31 However, the relation of obesity with osteoporosis has been questioned and several studies have supported an aggravating role of obesity in osteoporosis.32-35 Unfortunately, neither DEXA nor BIA-ACC® could estimate obesity-related qualitative changes of the muscle and bone architecture.
Even though we did not observe any significant differences in height between the three groups studied, height was negatively correlated with age, both in the total group and separately in the study groups. This finding confirmed previous studies.36
Presence of sarcopenia correlated strongly with the decreased bone density T-score groups in our study. Numerous earlier and recent studies support a “synergistic” role of sarcopenia in the development of osteoporosis, as well as bone fracture incidence.37-40 One general major limitation is the lack of definition of sarcopenia. No consensus exists and no task force has as yet defined universally acceptable cut-offs; however, extrapolating from the osteopenia (T-score <1SD - <2.5SD) and osteoporosis (<2.5SD) cut-offs would be a reasonable approach. Interestingly, DEXA operating programs do not include automated results of skeletal muscle mass reports and thus do not provide sarcopenia values.23 One should note that the formulas used in DEXA for sarcopenia estimation lack accuracy, as sarcopenia is an uneven condition, disturbing mainly postural muscles, while DEXA underestimates limb body mass by up to 20%.41 Only one study used mathematical predictions of sarcopenia in a sample of an aged population.42 This was the first prediction model used to identify sarcopenia based on parameters of demographic and functional fitness measures in community-dwelling older adults. Interestingly, Drey at al supported the combined management of decreased bone density together with muscle mass rehabilitation, as sarcopenia is a strong risk factor for falls and bone fractures.37
We have also observed interesting correlations between fat, skeletal muscle and bone mass in the total sample. These correlations confirm previous studies that have disputed the value of weight loss in postmenopausal women granted that in the absence of physical activity the loss may involve not only fat but skeletal muscle and bone mass.43,44 Our findings indicate the negative impact of estrogen decrease not only on bone mass, but also possibly on other tissues that derive from mesenchymal stem cells, such as the skeletal muscles.45 Increased fat mass was associated with increased IMAT in our subjects, suggesting suboptimal skeletal muscle functioning in the more obese individuals. As previously reported, elevated inflammatory adipocytokine secretion by increased fat mass is associated with the chronic mild “smoldering” systemic inflammation that is always associated with obesity.15
Most of our subjects exhibited low adherence to physical activity. This finding is similar to that of a previous observational study performed in the Attica prefecture, Greece, in 2005.46 It may also be the reason behind the high prevalence of sarcopenia given that physical activity is a very important component in the management of patients with body weight problems and bone metabolic disorders.
MUS and feelings of guilt were increased in the groups with decreased T-scores and demonstrated no relation with age or BMI. MUS are physical symptoms whose presence, severity or consequences cannot be conclusively explained by any detectable physical disorder. MUS are more prevalent in females than males, in younger than in older subjects and in the currently employed than in the non-employed.47 MUS are quite common in later life; however, the available data suggest that the prevalence rate declines after the age of 65 years.48 These apparently contradictory results might be due to the lack of a consensus in the definition of MUS, which fall into the category of somatomorphic disorders, as well as the paucity of guidelines of MUS definition and management.49 Estimates of prevalence inevitably vary with definition, but are higher in patients seen at primary care doctor’s offices, with these physicians having more confidence in their ability to diagnose and manage mental health problems. Feelings of guilt are associated with other depressive symptomatology,50 while recent studies have associated depression with bone fracture risk and mental health.51-53 Our BIA-ACC® platform results of the MUS questionnaire confirmed the aforementioned studies and provided an easy, reliable screening test in postmenopausal women (supplementary information).
DEXA has been employed as the “gold standard” method for body composition analysis. BIA-ACC® has the advantage over DEXA of avoiding radiation exposure. One strength of our study is the use of the two methods in conjunction to assess the reliability and agreement between them and not just to compare them by means of one statistical test, as a recent systematic review has suggested.28 The BIA-ACC® method has shown consistent repeatability in postmenopausal women.
There are limitations of our study. First, we did include the fat free mass of the BIA device in the indices tests. In BIA equations, since fat and fat free mass equals 1,13 it would be considered redundant. As mentioned earlier, DEXA possibly underestimates fat free mass,41 while skeletal muscle mass biopsies are considered too invasive for research settings. We found that intramuscular adipose tissue infiltration correlated with BMI and total adipose tissue mass, suggesting muscular degeneration in the more obese subjects, regardless of their muscle and bone mass. Lastly, we did not administer any psychometric questionnaire for stress, anxiety or depression in our subjects.
The acceptance and potential applications of diagnostic point-of-care testing (POC) in resource-limited settings, such as private practice offices, have been developing rapidly and are likely to continue growing. The goal of the introduction of new POC tests and POC devices is to provide fast, affordable test results to clinicians so that they may make an expedited clinical management decision and hence improve patient outcomes and overall public health.54-56 The BIA-ACC® device offers an acceptable determination of body composition with a satisfactory variability and with demonstration of a strong concordance with the traditional DEXA reference standard in postmenopausal women. It also provides information about sarcopenia and serves as a safe screening tool for abnormal bone density. Thus, this device provides screening and monitoring evaluations that are important in the management of subjects with chronic fat, muscle and bone metabolic disorders.
Future studies should be performed to validate the diagnostic potential of BIA-ACC® devices in other age and gender groups and in subjects with pathologies affecting fat, muscle and bone mass.
ACKNOWLEDGEMENTS
We thank the nurse coordinator Melpomeni Veloni for her invaluable help and support in the recruitment of the participants. We also thank Dr. George Paltoglou, MD, PhD for his contribution to the figure quality.
CONFLICT OF INTEREST
Melpomeni Peppa, Charikleia Stefanaki, Athanasios Papaefstathiou, George Dimitriadis and George P. Chrousos have no conflict of interest that could be perceived as prejudicing the impartiality of the research reported. Dario Boschiero is the director of BIOTEKNA s.r.l., Venice, Italy, that provided the BIA-ACC® device for this study.
FUNDING
BIOTEKNA Co, Venice, Italy, provided the BIA-ACC® device for this study. This research was supported by the National and Kapodistrian University of Athens and did not receive any specific grant from any funding agency in the public, commercial or not-for-profit sectors.
REFERENCES
1. Burger HG, Dudley EC, Robertson DM, Dennerstein L, 2002 Hormonal changes in the menopause transition. Recent Progr Horm Res 57: 257-275.
2. Toth MJ, Tchernof A, Sites CK, Poehlman ET, 2000 Menopause-related changes in body fat distribution. Ann NY Acad Sci 904: 502-506.
3. Baccaro LF, Conde DM, Costa-Paiva L, Pinto-Neto AM, 2015 The epidemiology and management of postmenopausal osteoporosis: a viewpoint from Brazil. Clin Interv Aging 10: 583-591.
4. Van Pelt RE, Gozansky WS, Hickner RC, Schwartz RS, Kohrt WM, 2006 Acute modulation of adipose tissue lipolysis by intravenous estrogens. Obesity 14: 2163-2172.
5. Peppa M, Koliaki C, Dimitriadis G, 2012 Body composition as an important determinant of metabolic syndrome in postmenopausal women. Endocrinol Metabol Syndrome S1: 009.
6. Peppa M, Koliaki C, Boutati E, Garoflos E, Papaefstathiou A, Siafakas N et al, 2014 Association of lean body mass with cardiometabolic risk factors in healthy postmenopausal women. Obesity (Silver Spring) 22: 828-835.
7. Peppa M, Koliaki C, Papaefstathiou A, Garoflos E, Katsilambros N, Raptis SA et al, 2013 Body composition determinants of metabolic phenotypes of obesity in nonobese and obese postmenopausal women. Obesity 21: 1807-1814.
8. Kanellakis S, Manios Y, 2012 Validation of five simple models estimating body fat in white postmenopausal women: use in clinical practice and research. Obesity 20: 1329-1332.
9. Wang J, Thornton JC, Kolesnik S, Pierson RN, Jr., 2000 Anthropometry in body composition. An overview. Ann N Y Acad Sci 904: 317-326.
10. Alokail MS, Sabico S, Al-Saleh Y, et al, 2013 Effects of probiotics in patients with diabetes mellitus type 2: study protocol for a randomized, double-blind, placebo-controlled trial. Trials 14: 195.
11. Elisha B, Rabasa-Lhoret R, Messier V, Abdulnour J, Karelis AD, 2013 Relationship between the body adiposity index and cardiometabolic risk factors in obese postmenopausal women. Eur J Nutr 52: 145-151.
12. Wang ZM, Pierson RN, Jr., Heymsfield SB, 1992 The five-level model: a new approach to organizing body-composition research. Am J Clin Nutr 56: 19-28.
13. Tsigos C, Stefanaki C, Lambrou GI, Boschiero D, Chrousos GP, 2015 Stress and inflammatory biomarkers and symptoms are associated with bioimpedance measures. Eur J Clin Invest 45: 126-134.
14. Bosy-Westphal A, Muller MJ, 2015 Identification of skeletal muscle mass depletion across age and BMI groups in health and disease—there is need for a unified definition. Int J Obes (Lond) 39: 379-386.
15. Stefanaki C, Peppa M, Boschiero D, Chrousos GP, 2016 Healthy overweight/obese youth: early osteosarcopenic obesity features. Eur J Clin Invest 46: 767-778.
16. Rutjes AW, Reitsma JB, Vandenbroucke JP, Glas AS, Bossuyt PM, 2005 Case-control and two-gate designs in diagnostic accuracy studies. Clin Chem 51: 1335-1341.
17. Simera I, Moher D, Hirst A, et al, 2010 Transparent and accurate reporting increases reliability, utility, and impact of your research: reporting guidelines and the EQUATOR Network. BMC Medicine 8: 24.
18. Binkley N, Bilezikian JP, Kendler DL, et al, 2007 Summary of the International Society for Clinical Densitometry 2005 Position Development Conference. J Bone Metab 22: 643-645.
19. Aleksic J, Alexa A, Attwood TK, et al, 2014 An open science peer review oath F1000Res 3: 271.
20. Begley CG, Ioannidis JP, 2015 Reproducibility in science: improving the standard for basic and preclinical research. Circ Res 116: 116-126.
21. Peppa M, Koliaki C, Hadjidakis DI, et al, 2013 Regional fat distribution and cardiometabolic risk in healthy postmenopausal women. Eur J Intern Med 24: 824-831.
22. Jackson AS, Blair SN, Mahar MT, et al, 1990 Prediction of functional aerobic capacity without exercise testing. Med Sci Sports Exerc 22: 863-870.
23. Edwards MH, Buehring B, 2015 Novel approaches to the diagnosis of sarcopenia. J Clin Densitom 18: 472-477.
24. Janssen I, Heymsfield SB, Ross R, 2002 Low relative skeletal muscle mass (sarcopenia) in older persons is associated with functional impairment and physical disability. J Am Geriatr Soc 50: 889-896.
25. McHugh ML, 2012 Interrater reliability: the kappa statistic. Biochem Med (Zagreb) 22: 276-282.
26. Atiqi R, van Iersel C, Cleophas TJ, 2009 Accuracy assessments of quantitative diagnostic tests for clinical research. Int J Clin Pharmacol Ther 47: 153-158.
27. Giavarina D, 2015 Understanding Bland Altman analysis. Biochem Med (Zagreb) 25: 141-151.
28. Zaki R, Bulgiba A, Ismail R, Ismail NA, 2012 Statistical methods used to test for agreement of medical instruments measuring continuous variables in method comparison studies: a systematic review. PLoS One 7: e37908.
29. Maimoun L, Mura T, Leprieur E, et al, 2016 Impact of obesity on bone mass throughout adult life: Influence of gender and severity of obesity. Bone 90: 23-30.
30. El Maghraoui A, Sadni S, El Maataoui A, et al, 2015 Influence of obesity on vertebral fracture prevalence and vitamin D status in postmenopausal women. Nutr Metab (Lond) 12: 44.
31. Migliaccio S, Greco EA, Aversa A, Lenzi A, 2014 Age-associated (cardio)metabolic diseases and cross-talk between adipose tissue and skeleton: endocrine aspects. Horm Mol Biol Clin Investig 20: 25-38.
32. Freitas PM, Garcia Rosa ML, Gomes AM, et al, 2015 Central and peripheral fat body mass have a protective effect on osteopenia or osteoporosis in adults and elderly? Osteoporos Int 27: 1659-1663.
33. Chung JH, Hwang HJ, Shin HY, Han CH, 2015 Association between sarcopenic obesity and bone mineral density in middle-aged and elderly Korean. Ann Nutr Metab 68: 77-84.
34. Butterworth PA, Menz HB, Urquhart DM, et al, 2016 Fat mass is associated with foot pain in men: The geelong osteoporosis study. J Rheumatol 43: 138-143.
35. Greco EA, Lenzi A, Migliaccio S, 2015 The obesity of bone. Ther Adv Endocrinol Metab 6: 273-286.
36. Hermanussen M, Scheffler C, Groth D, Assmann C, 2015 Height and skeletal morphology in relation to modern life style. J Physiol Anthropol 34: 41.
37. Drey M, Sieber CC, Bertsch T, et al, 2015 Osteosarcopenia is more than sarcopenia and osteopenia alone. Aging Clin Exp Res 28: 895-899.
38. Laurent MR, Dubois V, Claessens F, et al, 2015 Muscle-bone interactions: From experimental models to the clinic? A critical update. Mol Cell Endocrinol 432: 14-36.
39. Hamdy RC, 2015 Osteoporosis, Sarcopenia, falls and fractures: In this issue. J Clin Densitom 18: 447-448.
40. Perez-Lopez FR, Ara I, 2016 Fragility fracture risk and skeletal muscle function. Climacteric 19: 37-41.
41. Ryan AS, Dobrovolny CL, Smith GV, Silver KH, Macko RF, 2002 Hemiparetic muscle atrophy and increased intramuscular fat in stroke patients. Arch Phys Med Rehabil 83: 1703-1707.
42. Gray M, Glenn JM, Binns A, 2016 Predicting sarcopenia from functional measures among community-dwelling older adults. Age (Dordr) 38: 22.
43. Shea KL, Gozansky WS, Sherk VD, et al, 2014 Loss of bone strength in response to exercise-induced weight loss in obese postmenopausal women: results from a pilot study. J Musculoskelet Neuronal Interact 14: 229-238.
44. Von Thun NL, Sukumar D, Heymsfield SB, Shapses SA, 2014 Does bone loss begin after weight loss ends? Results 2 years after weight loss or regain in postmenopausal women. Menopause 21: 501-508.
45. Breu A, Sprinzing B, Merkl K, et al, 2011 Estrogen reduces cellular aging in human mesenchymal stem cells and chondrocytes. J Orthop Res 29: 1563-1571.
46. Panagiotakos DB, Pitsavos C, Zeimbekis A, Chrysohoou C, Stefanadis C, 2005 The association between lifestyle-related factors and plasma homocysteine levels in healthy individuals from the “ATTICA” Study. Int J Cardiol 98: 471-477.
47. Nimnuan C, Hotopf M, Wessely S, 2001 Medically unexplained symptoms: an epidemiological study in seven specialities. J Psychosom Res 51: 361-367.
48. Hilderink PH, Collard R, Rosmalen JG, Oude Voshaar RC, 2013 Prevalence of somatoform disorders and medically unexplained symptoms in old age populations in comparison with younger age groups: a systematic review. Ageing Res Rev 12: 151-156.
49. de CWAC, Cella M, 2012 Medically unexplained symptoms and pain: misunderstanding and myth. Curr Opin Support Palliat Care 6: 201-206.
50. Engel-Yeger B, Muzio C, Rinosi G, et al, 2016 Extreme sensory processing patterns and their relation with clinical conditions among individuals with major affective disorders. Psychiatry Res 236: 112-118.
51. Williams LJ, Pasco JA, Jackson H, et al, 2016 Depression as a risk factor for fracture in women: A 10 year longitudinal study. J Affect Disord 92: 34-40.
52. Zong Y, Tang Y, Xue Y, et al, 2016 Depression is associated with increased incidence of osteoporotic thoracolumbar fracture in postmenopausal women: a prospective study. Eur Spine J 25: 3418-3423.
53. Rauma PH, Koivumaa-Honkanen H, Williams LJ, et al, 2014 Life satisfaction and bone mineral density among postmenopausal women: cross-sectional and longitudinal associations. Psychosom Med 76: 709-715.
54. Drain PK, Hyle EP, Noubary F, et al, 2014 Diagnostic point-of-care tests in resource-limited settings. Lancet Infect Dis 14: 239-249.
55. Choi S, 2016 Powering point-of-care diagnostic devices. Biotechnol Adv 34: 321-330.
56. Engel N, Ganesh G, Patil M, et al, 2015 Point-of-care testing in India: missed opportunities to realize the true potential of point-of-care testing programs. BMC Health Serv Res 15: 550.
57. Lobo RA, Davis SR, De Villiers TJ, et al, 2014 Prevention of diseases after menopause. Climacteric 17: 540-556.
Address for correspondence:
Charikleia Stefanaki, MD, MSc, First Department of Pediatrics, National and Kapodistrian University of Athens, Thivon & Levadeias Str., Goudi, Athens 11527, Greece; Tel.: +30 2132013244, fax: +302132013237, E-mail: cstefanaki@gmail.com
Received:06-06-2017, Accepted: 12-06-2017