1Thyroid Cancer Canada/Cancer de la thyroϊde Canada, Toronto, Canada, 2Vivre sans Thyroϊde, Léguevin, France, 3Butterfly Thyroid Cancer Trust, Rowlands Gill, Tyne & Wear, UK, 4Bundesverband Schilddrüsenkrebs -- Ohne Schilddrüse leben e.V., Berlin, Germany, 5Light of Life Foundation, Manalapan, NJ, USA, 6Division of Epidemiology and Health Services Research, Institute of Medical Biostatistics, Epidemiology, and Informatics, University of Mainz, Mainz, Germany, 7Department of Nuclear Medicine, RWTH Aachen University Hospital, Aachen, Germany, 8Department of Nuclear Medicine, University Hospital Marburg, Marburg, Germany
OBJECTIVE: To comprehensively assess the experience of a large, diverse cohort and identify potential care improvements, the Thyroid Cancer Alliance, an international patient/survivor group coalition, surveyed thyroid cancer patients/survivors worldwide. DESIGN: English, German, French or Spanish versions of a self-developed 43-item questionnaire were completed, predominantly online, by 2398 respondents from the US (37.9%), Germany (21.3%), the UK (11.5%), Canada (11.4%), France (9%), and 35 other countries. Females and differentiated thyroid carcinoma patients each comprised ~87% of respondents. Diagnosis occurred at age 30-59 years in 71.8%, within <1 (1-5) year(s) before survey completion in 16.4% (55%). RESULTS: At diagnosis, no psychological (or other professional) support was offered to 92.6% (76.9%) of respondents, no patient organization referral was made to 84.1%, and no clear written disease/treatment information was given to 63%. The five leading care improvement suggestions involved increased informational/psychosocial support. Among respondents undergoing neck surgery pre-survey completion (n = 2380), 72.5% reported at least transient complications, including hypocalcemia (38.8%), voice problems (36.2%), numbness (28.7%), or restricted neck/shoulder movement (27.6%). CONCLUSIONS: This large, multinational, patient/survivor-initiated cross-sectional survey suggests that thyroid cancer patients/survivors have substantial unmet informational/psychosocial support needs and suffer frequent treatment morbidity; disease management and some patient/survivor experience differ appreciably among countries.
Hypothyroidism, International variation in treatment practices, Patient organizations, Patient support needs, Patient survey, Recombinant human TSH, Surgical complications, Thyroid cancer
INTRODUCTION
Scientific literature on thyroid malignancies generally has not comprehensively addressed patients’/survivors’ psychosocial needs during and after primary treatment, satisfaction with care, and perception of sequelae of therapeutic and diagnostic interventions. Rather, publications have largely focused on health-related quality-of-life scores regarding relatively limited areas,1 e.g., thyroid surgery, thyroid remnant ablation, or thyroid hormone therapy protocols or status;2 occasional publications have addressed psychological distress3-6 or illness perception.7 Further, study instruments have mostly been generic and psychologist-, physician- or nurse-designed and studies virtually all single-center, often with small sample size. Only a few qualitative investigations8-12 or individual narratives13,14 have more broadly explored the thyroid cancer patient/survivor experience.
To address this information gap, the Thyroid Cancer Alliance (TCA; www.thyroidcanceralliance.org), a coalition of national/regional thyroid cancer patient/survivor support organizations from Europe and the Americas, designed and conducted an international survey of patients/survivors with any primary thyroid tumor that sought to:
assess thyroid cancer life impacts in a large, diverse cohort
characterize recent thyroid cancer care in different countries
identify potential care improvements.
Nearly 2400 questionnaires were completed, including over 200 each from five large North American or European countries, representing, to our knowledge, the largest such survey yet published. We now report key survey findings using, for ease of reference, a traditional academic format, although this patient-driven project may be regarded as hypothesis-generating rather than as a formal scientific study.
SUBJECTS AND METHODOLOGY
Survey development and data collection
R.B., B.B., K.F., H.R. and J.S., respectively representing thyroid cancer patient/survivor groups in Canada, France, the UK, Germany, and the USA, consulting with fellow thyroid cancer patients/survivors, designed a comprehensive, anonymous self-report questionnaire (English version in Supplementary Appendix) containing 43 items, plus, depending on the answers to the items, up to 16 sub-items, all of which elicited categorical or quantitative data. Survey completion took ~15-20 min. The instrument was developed in English and translated into German, French, and Spanish. No formal questionnaire or translation validation was performed.
The survey was posted online in the four languages on a dedicated website, which was linked on thyroid cancer patient/survivor group websites. Patient/survivor organization members then were invited via e-mail, website notices or both to complete the questionnaire. Additionally, in the UK, the questionnaire was administered in person to patients in two thyroid cancer clinics and sent by post to patients from another such clinic. Data were collected in March 2010. A professional market research firm assisted with questionnaire development and data collection and analysis, while physician and psychologist advisors to the TCA (M.L., S.S., F.A.V.) helped interpret data. Because the survey was patient-initiated and –managed and anonymous, and entailed no diagnostic or therapeutic interventions, institutional review board/ethics committee approval was not sought.
Genzyme (Cambridge, MA, USA), the manufacturer of recombinant human thyroid-stimulating hormone (rhTSH; thyrotropin alfa, Thyrogen®), provided grant support for the project, as described elsewhere in this paper. The TCA controlled the survey instrument content and data, the choice and venues to publicize/publish the survey and survey data, and the content of any resultant manuscript(s) as well as of a poster presented at the XIV International Thyroid Congress regarding the survey results.
Statistics
Data analysis was descriptive. Only fully completed surveys were analyzed.
RESULTS
Sources of completed questionnaires
Of the 2398 fully completed questionnaires, 2195 (91.5%) were filled out online and 203 (8.5%) were completed at clinics or sent postally. The five countries with the most respondents were the USA (919, 37.9%), Germany (510, 21.3%), the UK (276, 11.5%), Canada (274, 11.4%), and France (217, 9%); 35 other countries accounted for the remaining 8.4% of participation.
Respondent characteristics (Table 1)
Females, younger patients, and those with metastases appeared to be over-represented among survey respondents relative to the general thyroid cancer patient population, and the large majority of respondents were diagnosed and treated in the early to mid-2000s.
Diagnosis
Thyroid cancer diagnosis was sometimes protracted. In ~70% of respondents, suspicion of thyroid cancer emerged due to suggestive signs/symptoms: discovery of a lump by the patient (39.4%) or family member (9.1%) or occurrence of respiratory/digestive tract symptoms (difficulty swallowing, 14.5%; difficulty breathing, 6.5%), or voice problems (hoarseness, 8.5%). In the remaining cases, evidence of disease was discovered incidentally in a routine check-up (24.8%) or on imaging for other indications (12.4%).
A ≥4-week wait was reported between referral and the first “thyroid specialist” physician visit in a little more than a quarter of respondents (Figure 1); long waits for the first specialist visit were especially common among Canadian, British, and French respondents (Table 2). The first “thyroid specialist” was an endocrinologist for 34.4% of respondents, a head/neck surgeon for 18.7%, a general surgeon for 18.5%, a nuclear medicine physician for 10.6%, an endocrine surgeon for 5.8%, and a physician from another specialty for 12% However, there were considerable national differences, with the most common initially consulted specialist being an endocrinologist in Canada, France, and the USA, but a nuclear medicine physician in Germany and a general surgeon in the UK (Table 2).

Figure 1. Reported wait times for first “thyroid specialist” visit. Percentage of all respondents (N = 2398) in various wait time ranges.
A ≥4-week wait between the first specialist visit and delivery of a thyroid cancer diagnosis was reported by 18.2% of respondents; long waits again were relatively more commonly noted among Canadian, British, and French respondents (Table 2).
Lack of psychosocial/informational support at diagnosis
As seen in Figure 2, the vast majority of respondents reported that at diagnosis, they were not offered assistance from a psychologist/counselor, nurse or other support professional, referral to a patient organization, or clear written information about their disease/treatment. Among the five nationalities with the most survey participation, this lack of support was most commonly reported by French respondents and least commonly by UK respondents (Table 2). Except in the case of psychologist/counselor assistance, a higher percentage of more recently diagnosed respondents reported being offered such support (data not shown).

Additional support from nurse or other support person 
Information on patient support organization 
Psychological support from a psychologist/counselor 
Clear written information on disease and treatment ![]()
Figure 2. Availability to respondents (N = 2398) of various forms of support and information at diagnosis.
Likely due to the limited availability of support, 85.4% of respondents sought information/support outside their clinic, from the internet (88.2% of those seeking outside resources), thyroid organization pamphlets (42.1%), patient groups (30.6%), books (30.3%), family physicians (28%), other thyroid cancer patients/survivors (27.5%), or friends/family (26.5%).
Surgery and its complications
Re-operation and post-surgical complications were frequently noted. Virtually the entire sample (2380, 99.2%) reported neck surgery for thyroid cancer before survey completion; the questionnaire did not query regarding surgical radicality. Of those undergoing neck procedures, 54.5% had one and 37.9%, two. Re-operation was less common more recently (56% of those diagnosed >5 years before survey completion, 44.4% of those diagnosed 1-5 years before, and 30.4% of those diagnosed <1 year before). Under 40% of respondents from France, Canada or the USA, but >60% from Germany or the UK, underwent more than one surgery (Table 3).
Of neck surgery recipients diagnosed ≥1 year before survey completion (n = 1995), 39.2% reported hypocalcemia, 35.6% voice problems, 28.9% numbness, and 26.8% restricted neck/shoulder/movement postoperatively (Figure 3A). Twenty-seven and a half percent of respondents reported no surgical complications. Time since surgery was not elicited.


Figure 3. Treatment side effects. Panel a): Reported post-surgical complications in respondents undergoing neck surgery for thyroid cancer diagnosed >1 year before survey completion (n = 1995). Right-hand percentages indicate the prevalence of a given complication in this subgroup. For each complication, the dark portion of the bars represents graphically, and the left-hand percentages provide values for, the proportion of respondents reporting that their complication was unresolved as of survey completion. Panel b): Reported side effects immediately after radioiodine treatment. Type of radioiodine treatment (therapeutic or diagnostic), activity, prophylactic measures against radiotoxicity, and persistence of side effects were not elicited by the survey. Values in the right-hand sections of the bars indicate the proportion of respondents reporting a given side effect of radioiodine treatment.
At least some postoperative symptoms remained unresolved in 1010 respondents, which corresponded to 57.9% of those noting complications (n = 1743) and 42.4% of those undergoing neck operations for thyroid cancer (n = 2380). Rates of unresolved surgical symptoms in respondents diagnosed ≥1 year before survey completion were high in all five countries with the largest survey samples, most so in Germany and Canada (Table 3). At least one third of respondents diagnosed ≥1 year before survey completion who reported hypocalcemia, voice problems, numbness, restricted neck/shoulder movement, or vocal cord palsy described the symptom(s) as persistent (Figure 3A); the survey did not define this term, however.
Radioiodine administration
Respondents frequently reported receiving radioiodine, in an appreciable percentage of cases, long after surgery; radioiodine side effects were commonly noted. Radioiodine was given to 83.9% (2011/2398) of respondents overall, including 87.3% of those with non-medullary thyroid cancer (n = 2293). Rates of radioiodine use were consistent across the five countries with the most respondents (Table 3). Of respondents receiving radioiodine, 69.1% did so as inpatients and 42.9% in more than one course; multiple activities were especially prevalent in Germany (Table 3). Notably, the survey did not explicitly distinguish between therapeutic versus diagnostic radioiodine use.
Just over 80% of respondents receiving radioiodine reported one or more side effects “immediately following” administration (Figure 3B). Radioiodine side effects were most frequently reported by Canadian and US respondents and least frequently by French and British respondents (Table 3). Most commonly noted, in descending order, were taste disturbance, dry mouth or salivary gland swelling or pain, each affecting more than a third of respondents receiving radioiodine, and nausea/vomiting or sore neck, each reported by more than a fifth of those respondents. The survey did not inquire about radioiodine side effect persistence.
The time between surgery and the first radioiodine course was >2 months in 28.6% of respondents receiving radioiodine, a situation reported especially commonly among Canadian respondents, but rarely among German respondents (Table 3). Using a 5-point Likert scale (1, very poor, to 5, very good), 62.1% of respondents receiving radioiodine rated the facility where their radioiodine was administered as good or very good and 8.8% as poor or worse.
TSH preparations and their side effects
Of survey respondents given radioiodine, 80.6% underwent thyroid hormone withdrawal (THW), lasting 4.1±2.2 weeks on average. Almost all patients undergoing THW (n = 1621) reported at least one hypothyroid symptom during thyroid hormone discontinuation. Depression was reported by 54.2% of respondents, inability to concentrate/think straight by 76%, and fatigue by 99.3%.
rhTSH was offered to 37.4% of survey respondents receiving radioiodine (752/2011), including 42% (26.5%) of those diagnosed within 5 (>5) years before survey completion. When rhTSH was offered, the option was almost always explained (91.4%) and almost always paid for by private or public health insurance or other third parties (92.7%). Notably, some respondents offered rhTSH may have declined it, while some not offered rhTSH nonetheless may have requested and received the drug; moreover, a number of respondents may have been given rhTSH for Tg testing only, a situation not covered by the question wording. Of 995 respondents answering yes/no regarding rhTSH side effects, a little over one-fifth noted such symptoms, most commonly fatigue (12.9%), headache (11.7%), nausea/vomiting (9.4%), and dizziness (7.3%). Of those expressing a preference (n = 915), 86.6% favored rhTSH, 2.6% THW, and 10.8% neither.
Follow-up care
As reflected by current caregivers of respondents diagnosed ≥1 year before survey completion (n = 2004), follow-up care continued to be overwhelmingly provided by specialists. Endocrinologists (53.3%), nuclear medicine physicians (14.9%), and oncologists (11.3%) increasingly took the lead, and surgeons (4.6%) far less frequently did so; other specialties followed the remaining 15.9% of patients. The increased role of oncologists and decreased role of surgeons was mainly seen in UK respondents (data not shown). A minority of overall respondents (24%) changed treatment centers. Among this subgroup (n = 575), just 21% attributed the switch to dissatisfaction with care, although another 16% cited as their rationale the need for more specialized care.
Assessment of the thyroid cancer experience
Receiving a cancer diagnosis (24.4%) and anxiety/uncertainty about the future (21.9%) were most commonly rated as the most difficult aspects of the thyroid cancer experience. Notably, 15.5% of respondents in total stated that lack of psychological/emotional (7.9%) or informational support (7.6%) was the most difficult aspect, while treatment side effects were rated as such by 11%.
Broadly speaking, suggestions for improving care (Figure 4) focused on five areas: 1) more information on the disease/treatment (first and fourth commonest suggestions, respectively cited by 45% and 34% of respondents); 2) introduction to patient groups or individual patients (third and fifth commonest suggestions, respectively cited by 42.5% and 26.8%); 3) psychologist or nursing support (second and seventh commonest suggestions, respectively cited by 43.1% and 16.6%); 4) quicker access to test results (sixth commonest suggestion, 23.9%); and 5) easier access to caregivers (eighth commonest suggestion, 16%).
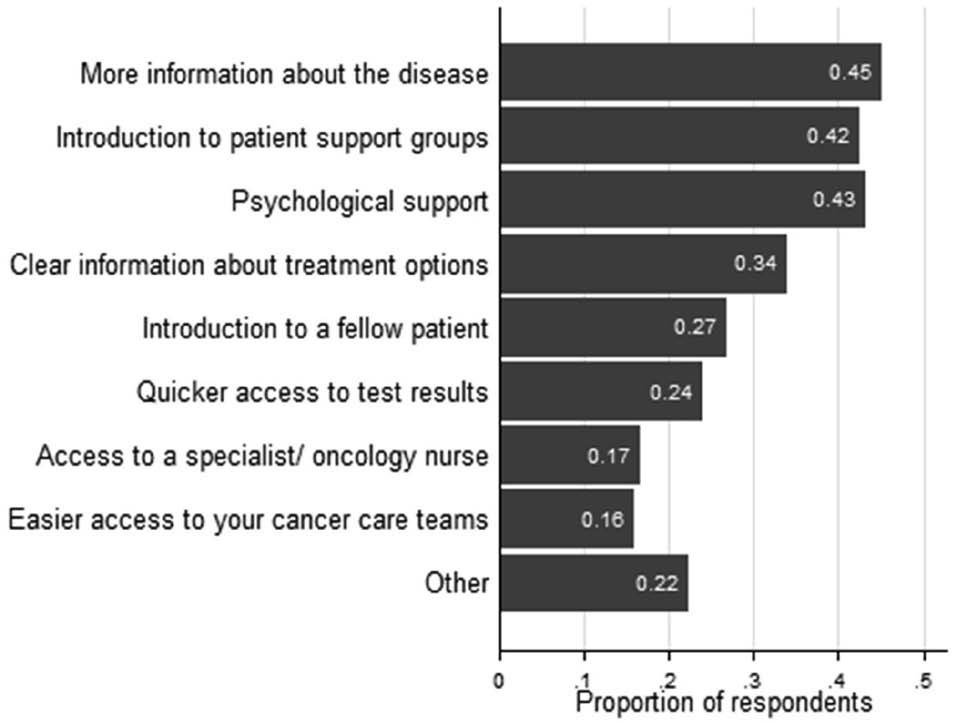
Figure 4. Improvements in care suggested by respondents (N= 2398). Values in the right-hand sections of the bars indicate the proportion of respondents suggesting a particular improvement.
DISCUSSION
This survey possessed important strengths: initiation, instrument design, and conduct by patients/survivors, a very large, geographically diverse response, resulting in, to our knowledge, the biggest cohort yet published and providing robust sample sizes for five large North American and European countries, and comprehensive content. However, the survey was not a formal scientific study, so its results are hypothesis-generating only.
Nonetheless, given the survey strengths and the frequency and consistency of certain responses, the thyroid cancer-treating community should regard the data reported here not only as stimulating further quality-of-care-related investigation, but as offering immediate opportunities to improve care. We believe that the survey findings can be grouped into three main themes meriting special attention.
First, thyroid cancer patients/survivors appear to have important unmet informational and psychological/emotional support needs (Figures 2 and 4, Table 2). This result accords with prior observations that among 1979 German university hospital cancer patients, those with thyroid malignancy received the least psycho-oncological support.15 In total, lack of information about the disease/treatment or lack of psychological/emotional support were cited directly by 15.5% of respondents as the hardest aspect of their thyroid cancer experience. Moreover, the most frequently cited difficulties, “receiving a cancer diagnosis” and “uncertainty/anxiety about the future”, together accounting for 46.2% of responses, may be attributable at least partly to a dearth of information/support. Presumably, these problems could be alleviated by increased access to fellow patients/survivors and counsellors as well as improved explanatory materials. In some settings, budgetary and logistical constraints may limit routine patient access to support professionals. However, many countries, including France, Germany, and the UK, include access to psycho-oncological care for patients in need as a main priority in National Cancer Plans, in certification guidelines for high-quality cancer centers, or both.16 Further, most institutions should be able to optimize “user-friendliness” and informativeness of in-house written materials for patients and significant others. “Off-the-shelf” resources represent another solution.17
The survey findings additionally suggest that patient organizations are an under-utilized informational/support source (Figure 2). These groups also represent a resource for improving written materials for patients/significant others.
Some thyroid cancer patients/survivors may forego counselling or contact with fellow patients/survivors. However, the high percentages of respondents advocating greater availability of psycho-oncological support and referrals to other patients/survivors (Figure 4) argue that these resources should at least be offered to every patient during initial treatment.
A second main theme of the survey findings was not infrequent long waits for first “thyroid specialist” visits, for the thyroid cancer diagnosis, and for post-surgical radioiodine treatment (Figure 1, Tables 2 and 3). Such delays likely would fuel the anxiety noted by survey respondents. Administrative solutions to this problem should be sought, but improved patient information/support should mitigate negative psychological/emotional effects.
Thirdly, survey findings highlight the apparent frequency of thyroid cancer treatment side effects (Figure 3). Respondents very often noted hypothyroid morbidity during THW, which, together with the important life impact of such symptoms, has been widely documented, e.g.;2,18-20 the expected relatively modest rhTSH side effects profile21,22 also was noted. However, reported rates of thyroid/neck surgery complications (Figure 3A, Table 3) and radioiodine side effects (Figure 3B, Table 3) seem high relative to published rates.11,23-25 Some difficulty exists in interpreting these findings, as for example, thyroid/neck surgery radicality, radioiodine activity, radiotoxicity prophylaxis, and treatment center type (e.g., referral versus community) were not elicited. We can only speculate regarding this discrepancy between our results and the literature. One possibility is that survey participants were more aware of their treatment toxicities, or experienced more symptoms, compared to the general thyroid cancer patient/survivor population; patients with treatment toxicities may tend to seek patient organizations and hence to have seen and completed this survey more often. An additional possibility is that participants in this patient-driven survey more honestly reported symptoms because they felt freer to express concerns, in contrast to patients in physician-led studies, who might hesitate to report problems in order not to burden or create conflict with their caregivers. Still another possibility relates to differences in timing of toxicity data collection relative to treatment, e.g., surgeons often see patients only once post-discharge and may focus their questions during that visit on incision healing. A fourth possibility is confounding effects due to recall bias: e.g., patients may lump together in their memory all aspects of their radioiodine therapy, including THW or rhTSH administration, the low-iodine diet period, and the actual radioiodine administration and its aftermath. An individual may develop a headache during the lead-up to therapy, which continues until thyroid hormone is re-instituted. Whether or not the headache was caused specifically by radioiodine ingestion is hard to separate in memory. Regardless of the explanation, our treatment toxicity-related findings should remind physicians to closely heed post-thyroidectomy serum calcium levels and to remember that radioiodine therapy is not free of e.g., salivary gland sequelae.
The survey also revealed interesting international variation in care patterns and the thyroid cancer patient/survivor experience (Tables 2, 3). These findings emphasize that not all treatment guidelines are internationally transferable26 and suggest the desirability of national quality improvement studies to identify local thyroid cancer patient care challenges.
Various survey limitations should be considered. For one, formal instrument validation was not performed. In hindsight, findings would have been strengthened by more precisely wording some questions, e.g., defining post-surgical symptoms and their baseline prevalence, or explicitly distinguishing diagnostic from therapeutic radioiodine use. Inclusion of certain follow-up questions, e.g., about initial TNM stage, neck surgery radicality, or radioiodine treatment side effect duration, also would have enhanced informativeness. Additionally, no mechanisms restricted response to thyroid cancer patients/survivors or to one per individual; however, little motivation seemingly would exist for survey completion by non-patients/survivors, or, except inadvertently, more than once per respondent. Another limitation, possibly related to the predominantly online data collection, was that respondents tended to be younger than is the general thyroid cancer patient population. To assess possibly resultant bias, we performed a post hoc exploratory analysis of differences among respondent age groups regarding supportive care needs and treatment side effects (data not shown). We found that 1) younger patients/survivors generally expressed more supportive care needs, 2) middle-aged patients more frequently reported being given sufficient information about patient groups or thyroid cancer or receiving psychological support, and 3) older patients oftener reported post-surgical voice problems, numbness, and low blood calcium. Given the apparent over-sampling of younger patients, the analysis suggests that the survey results might overestimate supportive care needs and underestimate the prevalence of post-surgery side effects in the general thyroid cancer patient population. Lastly, as an overall caveat, the descriptive nature of our data requires caution in interpreting results.
In conclusion, the TCA international thyroid cancer patient/survivor survey provides thought- and action-provoking data for thyroid cancer-treating healthcare professionals. Specifically, improvement in the clarity and comprehensiveness of written explanatory materials for patients and significant others, referrals to, and closer relationships with local and national patient/survivor groups, and identifying and preventing unwanted treatment effects should be prioritized. Additionally, administrative means should be sought to increase availability of psychological and other trained professional support for those in need and to decrease waits to see thyroid specialists, receive the thyroid cancer diagnosis, and undergo post-surgical radioiodine therapy.
ACKNOWLEDGMENTS
Survey participant organizations included: ACTIRA: Asociación Cáncer Tiroides República Argentina, Argentina (www.actiracancerdetiroides.blogspot.com); Butterfly Thyroid Cancer Trust, UK (www.butterfly.org.uk); Light of Life Foundation, USA (www.checkyourneck.com); Nordisk Thyreoidea Samarbeid, Scandinavia (www.geocities.com/tyreoideasamarbejd); Bundesverband Schilddrüsenkrebs -- Ohne Schilddrüse leben e.V., Germany (www.sd-krebs.de); ThyCa: Thyroid Cancer Survivors’ Association, Inc., USA (www.thyca.org); Thyroid Cancer Canada/Cancer de la thyroïde Canada, Canada (www.thyroidcancercanada.org); Vivre sans Thyroïde, France (www.forum-thyroide.net).
Portions of the data in this manuscript were reported by two of the authors (K.F., M.L.) in an oral presentation and included in a meeting poster at the XIV International Thyroid Congress, Paris, France, September, 2010. This patient survey was supported by a grant from Genzyme, a Sanofi Company, the recombinant human TSH manufacturer, furnished in the form of direct payments to certain vendors enumerated below. The grant included support of assistance in questionnaire development, data collection and analysis by a professional market research firm, Holden Pearmain Research, Weybridge, Surrey, UK. Also supported by the grant was editorial work by an independent medical editor, Robert J. Marlowe, Spencer-Fontayne Corporation, Jersey City, NJ, USA, on this manuscript and on the previously mentioned meeting poster. Additionally, the grant included payments to other firms providing logistical and meeting poster design/production support.
We thank Jamie Margerison and Alastair McDougall of Holden Pearmain Research for their support.
REFERENCES
1. Husson O, Haak HR, Oranje WA, Mols F, Reemst PH, van de Poll-Franse LV, 2011 Health-related quality of life among thyroid cancer survivors: a systematic review. Clin Endocrinol (Oxf) 75: 544-554.
2. Pitoia F, Licht S, 2007 Evaluation of the effect of thyroid hormone withdrawal on the quality of life of patients with differentiated thyroid carcinoma (in Spanish). Glánd Tir Paratir 16: 25-29.
3. Larisch R, Kley K, Nikolaus S, et al, 2004 Depression and anxiety in different thyroid function states. Horm Metab Res 36: 650-653.
4. Tagay S, Herpertz S, Langkafel M, et al, 2005 Health-related quality of life, anxiety and depression in thyroid cancer patients under short-term hypothyroidism and TSH-suppressive levothyroxine treatment. Eur J Endocrinol 153: 755-763.
5. Tagay S, Herpertz S, Langkafel M, et al, 2006 Health-related quality of life, depression and anxiety in thyroid cancer patients. Qual Life Res 15: 695-703.
6. Freyer G, Dazord A, Schlumberger M, et al, 1999 Psychosocial impact of genetic testing in familial medullary-thyroid carcinoma: a multicentric pilot-evaluation. Ann Oncol 10: 87-95.
7. Hirsch D, Ginat M, Levy S, et al, 2009 Illness perception in patients with differentiated epithelial cell thyroid cancer. Thyroid 19: 459-465.
8. Dow KH, Ferrell BR, Anello C, 1997 Balancing demands of cancer surveillance among survivors of thyroid cancer. Cancer Pract 5: 289-295.
9. Stajduhar KI, Neithercut J, Chu E, et al, 2000 Thyroid cancer: patients’ experiences of receiving iodine-131 therapy. Oncol Nurs Forum 27: 1213-1218.
10. McGrath PN, Fitch MI, 2003 Patient perspectives on the impact of receiving radioactive iodine: implications for practice. Can Oncol Nurs J 13: 152-163.
11. Sawka AM, Goldstein DP, Brierley JD, et al, 2009 The impact of thyroid cancer and post-surgical radioactive iodine treatment on the lives of thyroid cancer survivors: a qualitative study. PLoS ONE 4: e4191.
12. Hoftijzer HC, Heemstra KA, Corssmit EP, van der Klaauw AA, Romijn JA, Smit JW, 2008 Quality of life in cured patients with differentiated thyroid carcinoma. J Clin Endocrinol Metab 93: 200-203.
13. Shey J, 2001 Why I started the Thyroid Cancer Foundation. Cancer 91: 623-624.
14. Farnell K, 2010 Personal view. Thyroid cancer: doctor-patient partnership. Clin Oncol (R Coll Radiol) 22: 512-513.
15. Singer S, Hohlfeld S, Müller-Briel D, et al, 2011 Psychosocial care for cancer patients. Care provided and requirements. Psychotherapeut 56: 386-393.
16. Federation of International Pyscho-Oncology Societies, 2012 Psychosocial care in cancer care: situation in the countries represented within the Federation of Psycho-Oncology Societies. Psycho-Oncology, in press.
17. Van Nostrand D, Wartofsky L, Bloom G, Kulkarni KP (eds) 2010 Thyroid cancer: A guide for patients, second edition, Keystone Press, Pasadena, MD, USA; pp. 1-434.
18. Luster M, Felbinger R, Dietlein M, Reiners C, 2005 Thyroid hormone withdrawal in patients with differentiated thyroid carcinoma: a one hundred thirty-patient pilot survey on consequences of hypothyroidism and a pharmacoeconomic comparison to recombinant thyrotropin administration. Thyroid 15: 1147-1155.
19. Schroeder PR, Haugen BR, Pacini F, et al, 2006 A comparison of short-term changes in health-related quality of life in thyroid carcinoma patients undergoing diagnostic evaluation with recombinant human thyrotropin compared with thyroid hormone withdrawal. J Clin Endocrinol Metab 91: 878-884.
20. Dow KH, Ferrell BR, Anello C, 1997 Quality-of-life changes in patients with thyroid cancer after withdrawal of thyroid hormone therapy. Thyroid 7: 613-619.
21. Pacini F, Ladenson PW, Schlumberger M, et al, 2006 Radioiodine ablation of thyroid remnants after preparation with recombinant human thyrotropin in differentiated thyroid carcinoma: results of an international, randomized, controlled study. J Clin Endocrinol Metab 91: 926-932.
22. Haugen BR, Pacini F, Reiners C, et al, 1999 A comparison of recombinant human thyrotropin and thyroid hormone withdrawal for the detection of thyroid remnant or cancer. J Clin Endocrinol Metab 84: 3877-3885.
23. Mendoza A, Shaffer B, Karakla D, Mason ME, Elkins D, Goffman TE, 2004 Quality of life with well-differentiated thyroid cancer: treatment toxicities and their reduction. Thyroid 14: 133-140.
24. Thomusch O, Machens A, Sekulla C, et al, 2000 Multivariate analysis of risk factors for postoperative complications in benign goiter surgery: prospective multicenter study in Germany. World J Surg 24: 1335-1341.
25. Sherman SI, Brierley JD, Sperling M, et al, 1998 Prospective multicenter study of thyroid carcinoma treatment: initial analysis of staging and outcome. National Thyroid Cancer Treatment Cooperative Study Registry Group. Cancer 83: 1012-1021.
26. Dietlein M, Verburg FA, Luster M, Reiners C, Pitoia F, Schicha H, 2011 One should not just read what one believes: the nearly irresolvable issue of producing truly objective, evidence-based guidelines for the management of differentiated thyroid cancer. Eur J Nucl Med Mol Imaging 38: 793-798.
SUPPLEMENTARY APPENDIX: QUESTIONS
Q1. Which country do you live in?
Q2. Are you female ...
Q3. Do you have a family history of thyroid cancer?
Q3A. Have you had any genetic testing done for thyroid cancer?
Q3B. Has one or more of your blood relatives (family members) had genetic testing done for thyroid cancer?
Q4. How old were you when you were diagnosed with thyroid cancer?
Q5. How long is it since your diagnosis of thyroid cancer?
Q6. What led you to see your doctor initially?
Q7. How long did you wait to be seen by a thyroid specialist once you were referred?
Q8. Who was the first thyroid specialist that you saw when you were suspected of having thyroid cancer?
Q9. Which tests did you have done to evaluate your thyroid lump before you were diagnosed with thyroid cancer?
Q10. Did you have surgery to gain your diagnosis?
Q11. How long did you wait from seeing your specialist to getting your diagnosis?
Q11A. Please would you say exactly how long you waited?
Q12. Who told you that you had thyroid cancer?
Q13. How was the diagnosis given?
Q14. What type of thyroid cancer were you diagnosed with?
Q14A. Was this a familial/genetic type?
Q15. Were you offered any additional support at the stage of diagnosis from a specialist oncology/ cancer nurse or other support person?
Q16. Were you given a patient support organisation’s details at the stage of diagnosis?
Q17. Were you offered psychological support from a psychologist/counselor at the stage of diagnosis?
Q18. At the time of diagnosis were you given clear written information about your disease and its treatment?
Q19. Did you seek information/support from outside the hospital clinic at any point after you were diagnosed?
Q19A. Please could you say where you sought information or support?
Q19B. Which of these information sources did you find most beneficial to you personally?
Q20. How many neck surgeries have you had for thyroid cancer?
Q21. Please could you indicate if you experienced any of the following post surgery complications?
Q22. Have these post surgery complications now been resolved?
Q22A. Which of your post surgery complications have not been resolved yet?
Q23. Have you had radioactive iodine treatment?
Q24. How many radioactive iodine treatments have you had?
Q25. How long after surgery did you have your first radioactive iodine treatment?
Q25A. Please would you say how long it was before you had radioactive iodine treatment after your first thyroid cancer surgery?
Q26. Did you stop taking all thyroid hormone replacement in preparation for your radioactive iodine treatment?
Q26A. Please would you say how many weeks you stopped taking all thyroid hormone replacement?
Q27. Did you experience any symptoms of hypothyroidism when you stopped thyroid hormone replacement?
Q28. Did you experience any side effects immediately following RAI treatment?
Q29. Was your treatment given as an inpatient or outpaitent?
Q30. How did you find the facilities in the room where your treatment was given?
Q31. Did you seek help from your family doctor/ physician during the period before and/or after treatment?
Q32. Did you require antidepressants and/or sleeping tablets during this time?
Q33. Did your thyroid specialist offer rhTSH instead of withdrawal from thyroid hormone?
Q33A. Was the option of rhTSH explained to you?
Q34. If you had rhTSH did you experience any side effects?
Q34A. Please could you say what these side effects were?
Q34B. Please could you say who covered the costs for your treatment with rhTSH?
Q34C. Did this place you and/or your family under financial difficulty?
Q35. If you have experienced both rhTSH and withdrawal, please could you say which you preferred?
Q36. How long after radioactive iodine treatment did you feel able to return to your normal range of activities?
Q37. What is your current replacement hormone regime?
Q38. Who is currently responsible for your care?
Q38b. If you have changed hospitals/centres, please could you indicate the reason for this?
Q39. Are you currently disease free?
Q40. Do you have any metastatic disease outside the neck?
Q40A. How is this being managed?
Q41. Have you been given access to a clinical trial or ‘off label’ drugs?
Q41A. Could you tell us which one?
Q42. Overall what would you say was the most difficult aspect of your cancer journey?
Q43. What, if anything, could your medical team have done to improve this?
Address for correspondence:
Markus Luster, M.D., Department of Nuclear Medicine, Department of Nuclear Medicine,
University Hospital Marburg, Baldingerstrasse, 35043 Marburg Germany,
Tel.: +49-(0)6421/58 62815, Fax: +49-(0)6421/58 67025,
E-mail: markus.luster@med.uni-.-marburg.de
Received 02-08-2012, Accepted 24-01-2013